Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2021) Volume 37, Issue 6
Abiotic stresses have been recognized as the potential threat for agricultural production across the globe. Anthropogenic activities related to industrialization and urbanization also have aggravated the degradation of agricultural system as they are experiencing increasing impact of abiotic stresses. These stresses potentially induce various adverse effects on plants affecting their physiological, biochemical and molecular processes ultimately leading huge loss in crop productivity. Plant hormones are recognized amongst the handiest tools to mitigate the abiotic stress. Salicylic acid (SA) is one of most essential and multifaceted plant hormone that not only play vital role in plant defense but also have active participation in conferring abiotic stress tolerance. The present review deals with the illustrations of studies carried out by different workers on the role of SA in combating various types of abiotic stresses like metal stress, salinity stress, temperature stress and water stress in different crops.
Salicylic acid; Abiotic stress; Plant defense; Tolerance
Human beings are continuously exploiting natural resources to fulfill their needs without any check; this has huge negative impact on climatic conditions and agriculture. Though, there is improvement in agriculture production, but other natural resources are declining and when these coupled with increasing population, the overall situation appears alarming. Food and Agriculture Organization (FAO) predicted that by 2050, we need to enhance agricultural food production by 70% (FAO, 2009). The effects of changing climate like global warming showed direct effect on the plant production and as per the report of IPCC (2007) there will be increase in global temperature of earth by 2oC-4oC and this will further affect the agricultural productivity [1,2].
Any negative change in the existing climate is the major cause of abiotic and biotic stress observed in a particular region. Several abiotic stresses coupled with developmental activities like urbanization and industrialization have been assessed as the potential threats to the agricultural productivity [3]. The major abiotic stresses includes salinity stress, water stress (flooding and scarcity) metal/metalloid stress, temperature stress (extreme temperature), nutrient stress (deficiency and excess) are some major issues for world agriculture [4-7]. All these abiotic stresses potentially modulate physiological, biochemical and molecular mechanism in plants irrespective to their developmental stages and cause severe loss in the yield of crop plants [8,9].
Salicylic Acid (SA): brief history
The name of Salicylic Acid (SA) was derived from the Latin word Salix (willow tree). The bark and leaves of willow tree used by Americans, Indians and Greeks to cure aches and fever. It has been documented that Hippocrates for the first time administered a drug to relive pain of women during child birth and fever, it was salicylic acid. It was first isolated as glucoside of salicylic alcohol, salicin by a German scientist Johann Andreas Buchner. Later on it was reported from 36 other plants in addition to willow tree [10-13]. The phytohormone SA is a simple phenolic compound (C7H6O3) with an aromatic ring to which one carboxylic and one hydroxyl group are attached. The biosynthesis of this molecule takes place through shikimate pathway, which later branched as isochorimate pathway and phenylalanine pathway. The most common pathway in plants for SA synthesis is isochorismate pathway. About 90% of SA in plant is synthesized by this pathway. However, SA biosynthesis may also be accomplished by phenylalanine pathway [14- 16]. The rout of synthesis of SA is schematically presented in Figure1. Earlier, it was reported to be involved in various physiological processes like stimulation of root development, stomata closure, and reduced transpiration reversal of the effects of Abscisic Acid (ABA) (Davies) and regulation of gravitropism [17]. Yuan and Lin (2008) suggested that deficiency or very high concentration of SA increases the susceptibility of plants towards abiotic stress but moderate or optimal concentration (0.01 mM to 0.05 mM) of SA may be useful for abiotic stress tolerance.
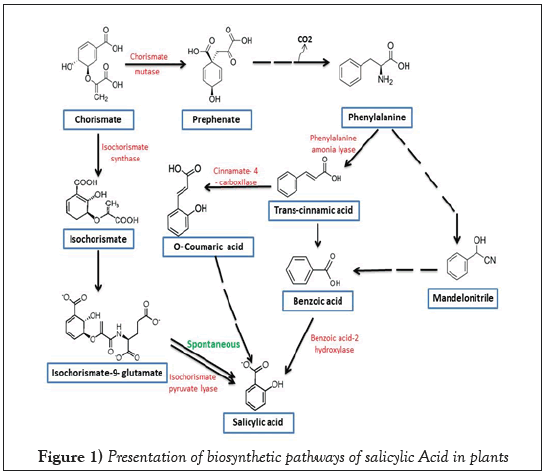
Figure 1: Presentation of biosynthetic pathways of salicylic Acid in plants
Salicylic Acid (SA) in abiotic stress tolerance
SA was reported to play a vital role in improving abiotic stress tolerance in several crop plants. Khan had taken an overview of historical background and biosynthesis of salicylic acid under both optimal and stressful environments in plants. They have also illustrated potential mechanisms governing salicylic acid-induced plant abiotic stress-tolerance. Li suggested that SA acts upstream of NO under high concentration of Carbon Dioxide (CO2) to induce enhanced flavonoid biosynthesis in tea plants. This indicates that SA enhanced biosynthesis of secondary metabolites in tea plants under the abiotic stress conditions. Zaid reported that in watermelon plants, resistance against root-knot nematode by red light is regulated by the coordination of SA and Jasmonic Acid (JA) signaling. This report indicates potential of SA in enhancing tolerance against biotic stress in watermelon. In general, it was noted to participate in several important plant processes and regarded as an important growth regulating and defense related molecules in plants [18- 20]. The overall role of SA in managing/combating different types of abiotic stress is been presented in Figure 2.
Figure 2: Presentation of mechanism of salicylic Acid in abiotic stress tolerance
SA in metal/metalloid stress tolerance
Plant responds to metals and metalloid stress by generating Reactive Oxygen Species (ROS). Overproduction of ROS can damage the nucleic acid, proteins, carbohydrates and lipid peroxidation [21,22]. To combat the uncontrolled oxidation, defense systems have to produce several enzymes (SOD, CAT, GR, GPX, GST etc.) and SA reportedly involved in this mechanism. SA increases plants tolerance to metal stresses by modulating various metabolites of antioxidant defense system, osmolytes, secondary metabolites and metal chelating compounds [23,24].
Some studies have reported that SA perform the role of an iron-chelation molecule or sometimes directly act as a scavenger of hydroxyl radicals and degrade metal-bioactivity by enhancing antioxidant enzyme activities [25]. Oxidative stress caused by ROS products such as H2O2 can be reduced by optimal concentrations of SA. In plants, when H2O2 is accumulated in high concentration, toxicity in plants caused by the production of reactive hydroxyl radicals can lead to oxidative stress and eventually disturb plant metabolism [26-30]. However, application of the low concentration of SA is reported to improve plant tolerance and increase plant defense by inducing antioxidant enzyme activities, leading to reduced oxidative stress [31]. Exogenous application of SA was reported to improve growth and photosynthetic traits in several crop plants including lead (Pb) exposed Oryza sativa, Cd exposed Zea mays El Dakak RA. and Cu-exposed Phaseolus vulgaris [18]. The efficacy of SA as foliar supplement on two mentha cultivars, namely, Kosi and Kushal grown under Cd (50 μM) stress conditions was tested by Zaid [32-34]. Out of three foliar applications of plant growth regulators, the application of SA at different growth stages proved best in alleviating Cd toxicity. Application of SA was also found to tolerate as toxicity in two varieties of Artemisia annua L. [35]. This study also suggested that the application of SA via leaf significantly increased the artemisinin content in both varieties. Es-sbihi studied the effects of SA on physiological traits, distribution of glandular hairs and Essential Oil (EO) composition in Salvia officinalis L., grown on Cu contaminated medium and noted that its foliar spray enhance the synthesis of essential oil. Thus, exogenous application of SA could enhance plants tolerance against metal stress. In general, SA is one of most essential phytohormone that regulates the plants tolerance to abiotic stress especially metal stress by stimulating genes associated with expression of defense system, modulating the cellular redox homeostasis and alteration of transcription element activities [36]. Some of the important reports of alleviation of metal and metalloid stress using SA are presented in Table 1.
| Plant name | Conc. of salicylic acid used | Parameters studied | Response | References (author name & Year) |
|---|---|---|---|---|
| Cd stress | ||||
| Ricinus communis | 0.5 mM | Gas exchange, Chlorophyll content | - | Liu et al., 2011 |
| Brassica juncea | 1.0 mM | Mineral nutrient content | + | Ahmad et al., 2011 |
| Glycine max | 120 mM | SOD activity, GSH content, heme- oxygenase-1 activity, and Chlorophyll content, relative protein content | + | Noriega et al., 2012 |
| Poa pratensis | 0.5 mM | Nutrient element content | + | Guo et al., 2013 |
| Poa pratensis | 0.5 mM | Cd uptake | - | Guo et al., 2013 |
| Cucumis melo | 0.1 mM | Photosynthetic capacity, efficiency of PSII, Water use efficiency | + | Zhang et al., 2015 |
| Zea mays | 0.5 mM | Defense compounds | + | El Dakak and Hassan, 2020 |
| Nickel stress | ||||
| Thlaspi goesingense | 0.5 mM | Induction of SAT/ increase in GSH | + | Freeman et al., 2015 |
| Catharanthus roseus | 10-5 M | Content of alkaloids vincristine and vinblastine | + | Idrees et al., 2013 |
| Iron stress | ||||
| Arachis hypogaea | 1.0 mM | Fe uptake and balance of mineral nutrients | + | Kong et al., 2014 |
| Copper stress | ||||
| Phaseolus vulgaris | 0.5 mM | Photosynthetic traits and growth | + | Zhengin, 2014 |
| Manganese stress | ||||
| Cucumis sativus | 100 µM | Oxidative stress in exposed leaves | - | Shi and Zhu, 2008 |
| Mercury stress | ||||
| Vallisneria natans | 100 µM | Pb uptake and Mn, Ca, and Fe content | - | Wang et al., 2011 |
| Salinity stress | ||||
| Cucumis sativus | 1.0 mM | uptake of N, P, K, Ca, and Mg | + | Yildirim et al., 2008 |
| Zea mays | 0.5 mM | K+/Na+ and Ca2+/Na+ ratios | + | Tufail et al., 2013 |
| Vigna radiata | 0.5mM | Glycinebetaine (GB) production, net photosynthesis, plant dry mass | + | Khan et al., 2014 |
| Torreya grandis | 0.5 mM | Chlorophyll content, net CO2 assimilation rates, proline content | + | Shen et al., 2014 |
| Glycine max | 0.5 mM | Na+/K+ ratio | - | Ardebili et al., 2014 |
| Glycine max | 0.5 mM | SOD activity, ascorbate content | + | |
| Hardeum vulgare | 0.05 mM | MDA content, Na+/K+ ratio | - | Fayez and Bazaid, 2014 |
| Hardeum vulgare | 10-4 mM | Content and activity of Rubisco, Rubisco activase | + | Lee et al., 2014 |
| Brassica juncea | 0.5 mM | S assimilation | + | Nazar et al., 2015 |
| Gossypium barbadense | 200 ppm | Growth and yield characters | + | El- Beltagi et al., 2017 |
| Pennisetum glaucum | 0.05mM | Physiological traits governing yield | + | Yadav et al., 2020 |
| Zea mays | 0.5 mM | Sugar contents, protein, proline, and activities of SOD, POD, CAT | + | Fahad and Bano, 2020 |
| Cold, Chilling & Freezing stress | ||||
| Musa acuminata | 0.5 mM | Ultrastructure of chloroplast of mesophyll cells, mitochondria of mesophyll cells | + | Kang et al., 2007 |
| Hardeum vulgare | 0.1 mM | Apoplastic antioxidative enzymes, ice nucleation activity, pattern of apoplastic proteins | + | Mutlu et al., 2013 |
| Citrus limon | 2.0 mM | Total phenolics, activity of phenylalanine ammonialyase (PAL) | + | Siboza et al., 2014 |
| Spinacia oleracea | 1.0 mM | Trehalose, proline, tocopherol, ascorbic acid, osmolytes and antioxidants | + | Min et al., 2019 |
| Heat stress | ||||
| Brassica juncea | 1.0 mM | Accumulation of essential elements | + | Ahmad et al., 2011 |
| Triticum aestivum | 0.5 mM | Proline content, glutamyl kinase activity, gas exchange, water potential | + | Khan et al., 2013 |
| Triticum aestivum | 0.5 mM | Antioxidants | + | Karpets et al., 2020 |
| Water stress (Drought) | ||||
| Triticum aestivum | 1,2,3 mM | Nitrogen assimilation | + | Singh and Usha, 2003 |
| Triticum aestivum | 1.0 mM | Ca, Mg, and K in shoot and roots | + | Al Tayeb and Ahmed, 2010 |
| Zea mays | 0.001 mM | Leaf rolling degree, water potential, dry weight | + | Saruhan et al., 2012 |
| Simarouba glauca | 0.05 mM | Polyphenol, alkaloids, flavonoid content | + | Awate and Gaikwad, 2014 |
| Portulaca oleracea | 0.5 mM | growth, photosynthetic pigment contents, gas exchanges traits, fatty acid contents, compatible solutes, secondary metabolites | + | Saheri et al., 2020 |
| Triticum aestivum | 1.0 mM | Photosynthesis performance, membrane permeability, stress proteins and antioxidants | + | Khalvandi et al., 2021 |
Table 1: Some representative report on Salicylic Acid (SA) mediated response towards abiotic stress impact in wild as well as crop plants (Adopted partially from Khan et al., 2015)
SA in salinity stress tolerance
Increasing salt concentration in soil is a major issue causing salt or salinity stress in most part of the world. The major adverse effects of salinity stress include increased ion toxicity, osmotic stress, and nutrient acquisition, impaired stomatal conductance, reduction in leaf water potential, altered physiological/biochemical processes, and elevated ROS-causing oxidative stress [37-39]. The role of SA in strengthening salinity stress-tolerance mechanisms has been extensively evidenced in many crops including Brassica juncea and V. radiata [40-42].
Exogenous application of SA increases the proline content in wheat seedlings under salinity stress, thereby alleviating the deleterious effects of salinity [43]. The pre-treatment SA was found to improve the photosynthetic efficiency, enhanced Ascorbate Peroxidase (APX) and Guaiacol Peroxidase (GPX) activity in roots, and induces an accumulation of polyamines that could be correlated with increased tolerance to salinity [44,45]. The pre-soaking treatment of seeds with SA was reported that it positively affected the osmotic potential, shoot and root dry mass, K+/Na+ ratio and contents of photosynthetic pigments in wheat seedlings, under saline and non - saline conditions [46]. Salicylic acid promotes germination under saline conditions by reducing NaCl induced oxidative stress [47]. Bastam reported that the external application of SA enhanced the tolerance of pistachio seedlings to NaCl stress. Exogenous application of SA (0.5 mM) minimizes the negative effects of salt stress with evidence of increasing the growth and productivity of tomato plants which could be correlated with increased photosynthetic pigments, soluble carbohydrate, protein content, total proline and phenol, electrolyte leakage percentage and leaf relative water content of plants [48-50]. Boukraa also demonstrated that SA treatment (exogenous or endogenous) potentially alleviate the negative results of chickpea under salt stress by increasing content and activity of antioxidant enzymes.
Salicylic Acid (SA) mitigated salinity stress-injury in Solanum lycopersicum by causing characteristic changes in the expression pattern of GST gene family members [51,52]. SA was also reported to induce salinity tolerance and increased biomass in Torreya grandis as a result of increased chlorophyll content and the activity of antioxidant enzymes that eventually activated the process of photosynthesis and counter oxidative stress [53]. Foliar spraying of SA (0.5 mM) on mung been under salt stress condition induces accumulation of glycinebetaine due to increased methionine and suppressed ethylene formation under salt stress; this eventually enhances antioxidant system resulting in alleviation of adverse effects of salt stress on photosynthesis and growth [54].Some reports indicate that SA applications helped to combat salinity stress in various crops. Rajeshwari and Bhuvaneshwari had reviewed the role of salicylic acid in salinity stress tolerance in different crop plants.
In a hydroponic study exogenous SA was applied to alleviating salt stress in cucumber seedlings [55,56]. They noted that the exogenous application of SA alleviated the NaCl toxicity by enhancing photosynthesis and architecture of root system. Park Lee worked out the mechanism of SA and Sulfur (S) interplay under salt stress in mungbean plants. Salt-exposed plants showed an enhancement of reactive oxygen species (ROS), lipid peroxidase, glucose, antioxidant enzymes, reduced glutathione and proline but marked inhibitions in the nitrate reductase (NR), nitrite reductase (NiR) activities, Nitrogen content, photosynthesis rate, and growth traits. The supplementation of SA and S strengthened the antioxidant machinery, improved NR and NiR activities, Nitrogen content, antioxidant enzymes and also decrease accumulation of ROS and glucose (a photosynthesis repressor). This study suggested that proper application of SA with S scale down the negative impact of the NaCl-mediated changes in tested plants [57,58].
Liu and Miao studied the effect of foliar spray of SA on a maize hybrid grown in saline soil conditions. The salinity treatment was found to significantly increased sugar contents, protein, proline, and activities of SOD, POD, and CAT but decreased the chlorophyll, carotenoid, osmotic potential and membrane stability index. The external application of SA to plants grown under salt-stressed conditions further increases the osmolytes, antioxidant enzymes, contents of endogenous abscisic acid (ABA), indole acetic acid (IAA), root length, and fresh and dry weights of roots [59]. This report indicates that foliar application of SA proved to be effective in combating ill effects of salinity stress on maize. Some of the important reports countering salinity stress in crop plants using SA are presented [60-62].
SA in temperature (extreme) stress tolerance
Present scenario of temperature extremes i.e. high temperature (heat) and low temperature (cold/chilling/freezing) represent potential threats to the crop plants as both type of stress conditions could affect many physiological and biochemical processes and molecular mechanism directly or indirectly [63]. SA was noted to help out plants to counter heat stress and chilling stress [64].
Khan had shown that treatment of SA can alleviate heat stress in T. aestivum. In yet another study by Naeem exposed wheat plants to heat stress (40°C for 6 h) and studied the potential of 0.5 mM SA in alleviating the negative effects of heat stress on photosynthesis. Under heat stress, the net photosynthesis (Pn) and activity of ribulose 1,5-bisphosphate carboxylase (Rubisco) and photosynthetic nitrogen use efficiency (NUE) decreased, but metabolism of proline was found to be increased [65-68]. The results of this study suggested that SA supplementation alleviates heat stress effects by interacting with proline metabolism and ethylene formation to improve photosynthesis in wheat plants [69-72].
SA was also reported to protect ultra-structures in Musa acuminata seedlings under chilling stress [73-75]. Mutlu showed that external application of SA results in cold tolerance by enhancing antioxidant enzymes, ice nucleation activity, and the patterns of apoplastic proteins in H [76,77]. vulgare genotypes. SA mediated increased synthesis of total phenolics and the activity of PAL was reported to improve chilling tolerance in cold-stored lemon fruit [78-80]. In yet another related work, Kumar by using MALDI-TOF–TOF/MS showed that spraying 100 mM SA alleviates the heat-induced (38°C) oxidative stress damage in wheat plants via modulation of the expression of heat-stable genes and proteins.
Min studied the cellular mechanism of SA induced freezing tolerance in Spinach by metabolite profiling [81]. This study showed that the treated leaves showed presence of more trehalose, proline, tocopherol, ascorbic acid, higher amount of compatible solute (osmolytes) and antioxidants [82]. In a recent report, Pan worked out the involvement of hydrogen sulfide (H2S) in SA-induced chilling stress tolerance in cucumber seedlings by using specific scavenger. In this study, authors reported that SA acts as an up-streaming signaling molecule of cucumber plants by increasing antioxidant defense system and modulating the expression of chilling stress-responsive genes [83]. Few more studies indicating the role of SA in countering chilling, freezing or heat stress conditions are presented.
SA in water (flooding/scarcity) stress tolerance
The effect of SA on water stress was reported more pronounced as compare to other stresses. Some early reports showed that the SA treatment improve the response to drought stress [84-86]. The improved drought response induced by SA is mostly associated with an increase or maintenance of plant growth, Rubisco activity, and the ant oxidative capacity [87]. Miura also reported that SA is important to tolerate drought stress as it induces the expression of drought related genes. It was suggested that that salicylic acid might be act as ROS scavenger [88]. Jesus demonstrated that salicylic acid could potentially modulate physiological and hormonal changes in Eucalyptus globules under water deficit environment [89].
Now it is believed that the expressions of dehydrins, chaperones, protein kinase genes and rubisco genes are involved in countering ROS production in photosynthetically active tissues [90]. Few studies have shown that external application of SA results in a positive effect by protecting plants against the oxidative damage caused by drought stress [91,93]. As SA is involved in induction of various genes related to antioxidant system, it was also noted to be directly involved in water stress tolerance [94].
Foliar application of Si, SA and especially the combination Si+SA, markedly improved grain yield and yield components of the two wheat cultivars under water-deficit. The results of the study highlight the role of Si and SA application in regulating water-stress response in wheat plant, suggesting that Si and SA are involved in physiological activities that could cope the negative impact of water deficit [95]. Saheri studied the effect of foliar application of salicylic acid under drought stress in Portulaca oleracea L. Usually, drought stress showed a decrease in the photosynthetic pigments, gas exchanges attributes, growth, biomass production, soluble sugars, total phenolic, flavonoids and unsaturated fatty acids like oleic, linoleic and linolenic acid, stearic and behenic acid but increased the contents of H2O2, MDA, and palmitic and arachidonic acid, respectively.
Application of SA improved the growth, photosynthetic pigment contents, gas exchanges traits, fatty acid contents, compatible solutes, secondary metabolites and observed the decrease in level of drought-induced oxidative stress compounds. Similar reports were made by Zaid A et al. indicating the positive role of SA in drought tolerance of rice crops in sesame crop [96].
Fariduddin showed that SA treatments effectively ameliorated the negative effects of drought stress on wheat crop by improving the photosynthetic performance, keeping membrane permeability, induction of stress proteins, and enhancing the activity of antioxidant enzymes [97,98]. In the same year, with detailed morphological, physiological and biochemical alterations, Zhang Y. demonstrated that foliar application of SA improves water stress tolerance in Conocarpus erectus L. and Populus deltoides L. Some other reports on application of SA to tolerate water flooding conditions, water scarcity or drought conditions [99,100].
Abiotic stresses had been recognized as major threats to the agricultural production leading to huge loss in the productivity causing economic loss. To counter these, plants induce several physiological and molecular mechanisms. Investigations so far have shown that SA is a strong and potential tool in reducing and managing the adverse effects of most abiotic stresses on plants especially crop plants and their productivity. External application of SA has been shown to be beneficial for plants either in normal or stressful conditions. SA is reportedly regulating various plant metabolic processes and modulates the production of varied osmolytes, secondary metabolites and also maintains plant-nutrient status; and thus protect plants under abiotic stress conditions. Over last two decades, the work related to SA synthesis and its applications reaches to another level and revealed that a number of SA signaling components involved in a variety of cellular processes. Recent SA -centered discoveries have significantly increased the capabilities of this natural compound beyond its previously identified functions in local and systemic defenses, development, stress management, cellular repair, growth, senescence and programmed cell death. In the recent years researches on SA and its applications has given new dimensions. Current researches on SA include SA-mediated signaling processes in growth, development and stress management which have been elucidated under various environmental conditions. It’s important to investigate how SA induce and manage different types of abiotic stresses in various crop plants under different environmental conditions to improve the crop yield.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this review paper.
DK has designed and developed the concept, RG and SR wrote primary manuscript, RS has reviewed it with inputs and all authors read final manuscript and approved for publication.
This research work did not receive any specific grant from any funding agencies in the public, commercial, or not-for-profit sectors.
Citation: Koche D, Gandhi R, Rathod S, et al. An update on role of salicylic Acid (SA) in abiotic stress tolerance in crop plants: a review. AGBIR. 2021;37(6):219-225.
Received: 01-Nov-2021 Accepted: 15-Nov-2021 Published: 22-Nov-2021
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
