Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2024) Volume 40, Issue 3
Preserving the quality of tomatoes and extending their shelf life, due to their high perishability, is a major concern in the food industry, as consumers are increasingly wary of consuming foods with chemical additives. The aim of this study was to evaluate the effectiveness of hydroethanolic extracts from four leaves (Daniellia oliveri, Pouteria alnifolia, Tectona grandis and Thalia geniculata), used as vegetable food packaging, as natural tomato puree preservative. Evaluation of antioxidant activity using 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging showed that Tectona grandis exhibited the highest scavenging activity, followed by Pouteria alnifolia, Daniellia oliveri and Thalia geniculata, respectively. Antibacterial activity tests conducted on reference strains Escherichia coli Collection Institut Pasteur (CIP) 53126 and Staphylococcus aureus American Type Culture Collection (ATCC) 6538 revealed that S. aureus was more sensitive, with Minimum Inhibitory Concentration (MIC) values ranging from 1.25 to 2.50 mg/mL for the four plant extracts, compared to E. coli (2.5 mg/mL to 5 mg/mL). Tectona grandis extract exhibited the strongest antiradical activity, similar to ascorbic acid, followed by the extracts of Pouteria alnifolia, Daniellia oliveri and Thalia geniculata.
Physicochemical analysis of the tomato puree showed minimal pH variation and high enzyme inhibition in all samples containing the extracts, compared to the control, during the five-day storage period. This study suggests that the leaf extracts from the four plants studied can be successfully used in preserving tomato concentrate, with Tectona grandis extract showing the most pronounced preservative effect.
Antimicrobial; Anti-free radical; Anti-browning; Preservation; Plant extracts; Tomato
Food and nutrition security remains a significant challenge for developing countries, despite rapid population growth, post-harvest losses and the depletion of natural resources such as land and water due to climate change. Despite notable progress in increasing global food production, about half of the population in the Third World lacks access to sufficient food. This is due to various reasons, including the loss of food products, particularly fruits and vegetables. Quantitative and qualitative food losses of fruits and vegetables occur at all stages of the agricultural system, especially post-harvest. Fruits and vegetables could be one of the main options that can significantly contribute to food and nutrition security. Agricultural products based on fruits and vegetables, such as tomatoes are considered protective food. However, tomatoes are known to be a source of vitamins, minerals, antioxidants, dietary fiber and energy in the diet of populations that are essential to reduce the high rate of malnutrition. With a production of 266,685 tons in 2021, the tomato is one of the most important vegetable crops in Benin, contributing to economic growth, employment and poverty reduction. Unfortunately, these potentials are limited by high post-harvest losses. According to Islam et al., [1], the most significant problems related to tomato production in tropical and subtropical regions of the world are post-harvest losses and deterioration of nutritional quality. Post-harvest losses of fruits in developed countries are estimated at 5%-25%, while in developing countries, they are estimated at 20%-50%.
Enzymatic browning and microorganisms are major concerns in tomato preservation as they are responsible for changes in appearance, flavor, odor, texture and other qualities [2]. Limiting post-harvest losses of tomatoes remains a daily struggle for agri-food industries, particularly those in Benin. Nevertheless, to remedy these problems, means to fight against these microorganisms and browning have been put in place such as the use of chemical additives. Indeed, the use of synthetic chemicals to reduce postharvest losses of tomatoes and extend their shelf life is a threat to human and animal health and the environment. Many post-harvest treatment methods and technologies, such as cold storage (refrigeration), controlled or modified atmosphere storage and treatment with inhibitors of microorganisms and enzymes etc. have been developed over the years to extend shelf life and maintain tomato quality after harvest [3-5]. However, the difficult accessibility and high cost of these technologies for poor farmers is a concern for most developing countries. Efforts are currently being made to seek appropriate alternatives, such as organic antimicrobials, for pre-harvest management and for the reduction of post-harvest losses of fruits and vegetables. According to Mahmuda et al., [6], fruits treated with 40% neem leaf extract retain most of the quality parameters for a much longer period. Also, thirty-six (36) plant extracts were analyzed for their polyphenol content and antioxidant capacity to identify promising anti-browning agents [2]. In addition, in some countries to reduce the use of synthetic chemicals, many opted for vegetable leaf packaging not only because of its natural origin but also because it is biodegradable. Furthermore, it has been recognized that some leaves contain active compounds, aromatics, dyes, enzymes (e.g. papain) and antimicrobial agents (essential oils) that migrate from the plant leaf to the food product [7]. In Africa, the use of plant-leaf packaging, long established, was essentially a physical means of protecting food from external influences such as microorganisms, air and moisture [8]. However, the evaluation of the effects of leaf extracts used as food packaging on microbial and enzymatic activities to preserve the tomato puree and reduce postharvest losses is not addressed. The research and development of adequate and biological new techniques for tomato preservation, particularly tomato puree, is one of the main ways to achieve food security. Thus, the present study was carried out to evaluate the effect of leaf extracts used as food packaging on microbial and enzymatic activities in order to increase the shelf life of tomato puree.
Plant material and experimental set-up
Plant material consists of leaves from Daniellia oliveri, Pouteria alnifolia, Tectona grandis and Thalia geniculata. These leaves were harvested in Za-kpota (Zou department), Godohountin (Atlantic department) and Atrokpocodji (Littoral department) in Benin. The species were verified at the National Herbarium of the University of Abomey-Calavi. To prepare the leaves, they were dried in the laboratory under air conditioning at 18°C and then ground into a fine powder using an electric grinder (EXCELLA Mixer Grinder).
Extracts preparation
The powders of each species were extracted using a mixture of water and ethanol (80:20) through maceration for 2 h. The resulting mixtures were filtered using Whatman paper (Qualitative Circles 150 mm Cat No 1001 150). The filtrate was then concentrated under reduced pressure using a rotavapor. The concentrated extract was further dried in an oven at 40°C until complete evaporation. The hydroethanol extract obtained was stored at 4°C. The yields of the different extractions were calculated using the following formula
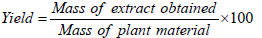
To assess the effects of various extracts on the shelf life of vegetables, specifically tomatoes, fresh and ripe tomatoes (Solanum lycopersicum L.) were obtained from the local market (Dantokpa) in Cotonou. The tomatoes were carefully washed and sorted and only those without any physical damage were selected. These selected tomatoes were then processed into puree using an electric crusher. The resulting puree was divided into five groups, each containing 300 mL of tomato puree.
The first group served as the control and remained untreated. The remaining four groups had extracts of Daniellia Oliveri, Pouteria alnifolia, Tectona grandiose and Thalia geniculata added to them at a concentration of 5 mg/mL. Each group was thoroughly mixed and divided into tubes, which were then sealed. The tubes containing the tomato purees were stored at room temperature (25°C ± 2°C) and used for various measurements and analyses on a daily basis over a period of five days. The entire experiment was conducted in triplicate to ensure accuracy and reliability of the results.
Phytochemical screening
Phytochemical analysis of the hydroethanol extracts was conducted to identify the presence or absence of various secondary metabolites, including alkaloids, coumarins, flavonoids, pigments, saponins, tannins, lignans, triterpenes, naphthoquinones and anthracene derivatives. Standard procedures outlined in Harborne [9] and Wagner et al., [10] were followed for the analysis. Standard procedures described by some authors were followed for the analysis [9,10].
Determination of total phenolics
Total polyphenols in extracts from various leaf species were quantified using the Folin Ciocalteu reagent method [11]. Stock solutions of the extracts were prepared in methanol at a concentration of 1 mg/mL. The reaction mixture consisted of 800 μl of Folin Ciocalteu 2 N reagent (Sigma Aldrich, F9252-100 mL) diluted to the 10th and 200 μl of the extract solution at 1 mg/mL. After a 5-minute incubation at room temperature, 1 mL of sodium carbonate solution (1 M) was added to the reaction medium, which was then incubated for 2 h at room temperature. Absorbance was measured at 765 nm using a spectrophotometer (UV-1600 PC). The results were expressed as milligrams of Gallic Acid Equivalents (GAE) per 100 milligrams of extract (mg GAE/100 mg extract).
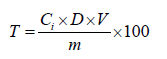
Where,
T=Total polyphenol content in mg GAE/100 mg dry matter; Ci=Concentration of sample read; D=Dilution factor; m=Mass of sample; V=Volume of sample
Determination of total flavonoids
The Aluminum Trichloride (AlCl3) method, as described by Nadhiya et al., [12], was employed to measure the total flavonoid content. The results were reported as milligrams of quercetin equivalent per 100 milligrams of extract (mg EQ/100 mg extract).
Evaluation of antioxidant activity
The antioxidant activity was assessed using the 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) test, following the spectrophotometric method outlined by Velazquez et al., [13]. A series of eight concentrations (ranging from 66 to 0.52 μg/mL) were prepared from a stock solution of the extract in methanol at a concentration of 0.2 mg/mL. These concentrations were used to evaluate the antiradical activity. The reaction mixture consisted of 0.75 mL of the extract and 1.5 mL of a 2% DPPH methanolic solution. A control sample, without any plant extract (sample blank), was prepared using methanol. The tubes were then incubated in the dark at room temperature for 15 min and the Optical Densities (OD) were measured at 517 nm. Ascorbic acid was used as a positive control. Each test was performed in triplicate. The concentration of the extract or fraction that caused a 50% inhibition of DPPH radicals in the reaction medium (IC50) was determined using the regression line equation obtained from the graph. The antiradical activity was calculated using the formula provided below:
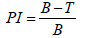
Where,
PI=Percentage inhibition; B=Sample-blank absorbance; T=Test absorbance
Evaluation of the antibacterial activity of the extracts
Microbial strains:The extracts were tested on microbial strains such as Escherichia coli CIP 53126 and Staphylococcus aureus ATCC 6538.
Pre-culture preparation:A pre-culture of microbial strains is prepared to obtain bacteria in the exponential growth phase. The Optical Density at 620 nm (OD 620) is then adjusted to 0.156 for Escherichia coli CIP 53126 strains and 0.3 for Staphylococcus aureus ATCC 6538, resulting in a bacterial inoculum of 108 CFU/mL. The final inoculum (106 CFU/mL) used for the assays is obtained by diluting the previous inoculum 1/100th to 1/100th.
Determination of minimum inhibitory concentration
The Minimum Inhibitory Concentration (MIC) of the extracts was determined using the liquid micro dilution method. This method helps identify the lowest concentration of extract that can inhibit bacterial growth. In this study, 100 μl of a bacterial inoculum with a concentration of 106 CFU/mL was added to all wells of a 96-well plate. Each well contained 100 μl of Muller Hinton (MH) broth and an extract concentration ranging from 5 to 0.039 mg/mL. To serve as controls, a negative control using a 50:50 acetone-water mixture and a positive control using a gentamicin concentration range of 5 to 0.039 mg/mL were included. The plates were then incubated at 37°C after the reaction media was homogenized. After 18 h of incubation, 40 μl of an indonitrotetrazolium solution (0.2 mg/mL) was added to each well and the plates were reincubated for 30 min. Wells that turned red/pink indicated bacterial growth, while wells that maintained their original color contained active extracts or fractions. All tests were conducted in triplicate to ensure accuracy. The determination of MIC is a reliable method for quantifying the activity of extracts.
Physicochemical analysis of tomato puree
Determination of pH:The pH of the tomato puree was measured using a previously calibrated digital pH meter (Eutech Instruments, pH 2700, Singapore). The change in pH (ΔpH) between days 0 and 5 of storage was calculated using the following formula:
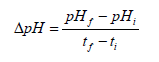
pHf and pHi represents the final and initial pH respectively; tf and ti represents the final and initial days respectively.
Evaluation of polyphenoloxidase activities
The Polyphenoloxidase (PPO) activity in tomato puree during the storage period was determined using Pizzocaro et al., [14] method with slight modifications. Two grams (2 g) of tomato puree were mixed with 2 mL of phosphate buffer solution (pH 6.5) and centrifuged at 3000 g and 4°C for 30 min. The resulting supernatant was used for PPO activity measurement at 25°C. To the 0.1 mL of the supernatant, 2 mL of catechol solution (0.1%) and 2 mL of buffer solution were added. PPO activity was measured in triplicate using a spectrophotometer (UV-visible 2600, Precision Science Instrument, Shanghai, China) at 420 nm. One unit of PPO was defined as the amount of enzyme in the extract that increased absorbance by 0.001 units per minute. The activity was expressed in units of PPO per minute and per gram (U/min/g) of tomato puree. The change in PPO activity (Δppo) between days 0 and 5 of storage, corresponding to enzyme inhibition, was calculated using the following formula:
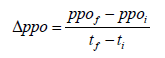
ppof and ppoi represents final and initial PPO activities, respectively; tf and ti represents final and initial days, respectively.
Evaluation of the color of tomato puree
Color is a crucial sensory characteristic when it comes to evaluating and selecting food products. The color measurements were conducted using a chromameter (CR-410, Konica Minolta Sensing, Tokyo, Japan) based on the color parameters defined by the International Commission on Illumination (CIE). These parameters are represented by three coordinates (L*, a* and b*) that describe each color. The color variation (ΔE) of the tomato puree was calculated using the following formula:
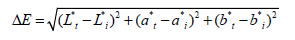
ΔE indicates the color variation between tomato puree Lt*, at* and bt* refer to individual instantaneous readings during the storage period and Li*, ai* and bi* refer to sample parameters on the initial day.
Microbiological analysis of tomato puree
All samples were tested for Total Aerobic Mesophilic Flora (TAMF) as well as yeast and mold growth during a 5-day storage period at room temperature. To analyze the samples, 1 mL of tomato puree was diluted one hundred times with distilled water. Then, 1 mL of the diluted sample was spread onto petri dishes containing the appropriate culture medium. TAMF was determined by counting the number of colonies on plaque count agar, while yeast and mold growth was assessed by measuring the diameter of the mycelium on potato dextrose agar. The incubation period for TAMF was 18 h at 35°C, while mold growth was observed for 120 h.
Statistical analysis
The results obtained from three separate experiments in our study were analyzed using Analysis of Variance (ANOVA) with Statistical Package for the Social Sciences (SPSS) 17.0 (SPSS Inc, USA). The statistical analysis of the results was performed using the student's t-test, which allowed us to calculate the Probability (P) value indicating whether a result showed a significant difference or not, with a significance level of 5% (p<0.05).
Extraction yield
The extraction yields of the different plant species are presented in Table 1. The yields vary depending on the type of leaves used. Pouteria alnifolia leaves exhibit the highest yield (21.50%), followed by Tectona grandis (15.64%), Daniellia oliveri (11.53%) and Thalia geniculata, which have the lowest yield (7.27%). Our findings differ from those of Onzo et al., [15], who reported extraction yields of 11.6% and 6.2% for Daniellia oliveri extracts and 6.4% and 5.6% for Thalia geniculata extracts using aqueous and hydroethanol extraction methods, respectively. The observed variations in extraction yields are likely attributed to the chemical composition of the leaves and the choice of extraction solvent. The extraction efficiency of a solvent depends on its affinity for phytomolecules and its polarity [16].
| Daniellia oliveri | Pouteria alnifolia | Tectona grandis | Thalia geniculata | |
|---|---|---|---|---|
| Yield (%) | 11.53 | 21.5 | 15.64 | 7.27 |
| Alkaloids | - | - | - | - |
| Coumarins | + | + | - | + |
| Anthracenic derivatives | - | + | + | + |
| Flavonoids | + | + | + | + |
| Lignans | + | - | + | + |
| Naphthoquinone | + | + | - | + |
| Pigments | - | + | - | + |
| Saponins | + | + | + | + |
| Tannins | + | + | + | + |
| Triterpenes | + | + | + | + |
Note: (+): Presence; (-): Absence.
Table 1: Yield and phytochemical of Daniellia oliveri, Pouteria alnifolia, Tectona grandis and Thalia geniculata extracts.
Phytochemical screening
The results of the phytochemical study of the extracts from the four different plant leaves (Daniellia oliveri, Pouteria alnifolia, Tectona grandis and Thalia geniculata) are presented in Table 1. The study revealed the presence of saponins, tannins, triterpenes and flavonoids in all four leaves. Additionally, coumarins and naphthoquinones were found in the hydroethanol extract of all leaves except Tectona grandis. Ligans were present in the extracts of Daniellia oliveri, Tectona grandis and Thalia geniculata, but absent in the extract of Pouteria alnifolia leaves. Anthracene derivatives were found in the leaves of Pouteria alnifolia, Tectona grandis and Thalia geniculata. Pigments were observed in the leaves of Pouteria alnifolia and Thalia geniculata. However, alkaloids were not detected in any of the leaf extracts from the four plants studied. These results align with previous studies by Tittikpina et al., [17] and Ogunmefun et al., [18], which also found flavonoids, tannins and saponins in the leaf extracts of Tectona grandis and Daniellia oliveri, except for alkaloids. This difference could be attributed to the choice of extraction solvent. Similarly, our findings are consistent with the work of Onzo et al., [19], who also reported the absence of alkaloids in Thalia geniculata extract, likely due to the extraction solvent used. Our results also share similarities with the phytochemical studies of the methanolic extract of Pouteria alnifolia stem bark Anyam [20].
The chemical compounds identified through phytochemical screening, including coumarins, flavonoids, pigments, saponins, tannins, triterpenes, naphthoquinones and anthracene derivatives, could explain the various traditional uses of these leaves in food packaging and for treating different diseases. These compounds are known to possess several beneficial properties such as anti-inflammatory, anti-hypertensive, anti-mutagenic, anti-coagulant, antioxidant, antiviral, immunostimulant, diuretic, antibacterial, antifungal, anti-tumor and anti-diarrheal effects [21,22].
Polyphenolic compounds
The Table 2 shows the total polyphenol and flavonoid contents of different plants. The total polyphenol content varies among the plants tested. The highest content was found in Pouteria alnifolia extracts (11.04 ± 0.28 mg EAG/100 mg), followed by Daniellia oliveri (9.50 ± 0.14 mg EAG/100 mg), Thalia geniculata (7.96 ± 0.62 mg EAG/100 mg) and Tectona grandis (5.04 ± 0.55 mg EAG/100 mg). These results differ from those reported by Degbe et al., [23] who found high levels for Tectona grandis (141 ± 44 mg EAG/100 mg) and low levels for Daniellia oliveri (3.99 ± 0.24 mg EAG/100 mg). The difference in total polyphenol content between our results and those of Degbe et al., [23] could be attributed to the different harvesting locations of Tectona grandis and the specific plant parts used for Daniellia oliveri.
| Plants extracts | Total polyphenol (mg EAG/100 mg) | Total flavonoid (mg EQ/100 mg) | IC50 |
|---|---|---|---|
| Daniellia oliveri | 9,50 ± 0,14c | 33,85 ± 1,70c | 5.85 |
| Pouteria alnifolia | 11,04 ± 0,28d | 13,99 ± 0,33b | 5.57 |
| Tectona grandis | 5,04 ± 0,55a | 6,79 ± 0,22a | 4.4 |
| Thalia geniculata | 7,96 ± 0,62b | 42,06 ± 4,44d | 7.87 |
Note: Values are means ± standard deviation (n=3). Data in same column with different letters are significantly different (p<0.05).
Table 2: Polyphenolic compounds and Inhibitory Concentration (IC50) of Daniellia oliveri, Pouteria alnifolia, Tectona grandis and Thalia geniculata extracts.
Regarding flavonoid content, it also varies among the different plants tested. Thalia geniculata exhibited the highest flavonoid content (42.06 ± 4.44 mg Equivalent Quercetin (EQ)/100 mg), followed by Daniellia oliveri (33.85 ± 1.70 mg EQ/100 mg), Pouteria alnifolia (13.99 ± 0.33 mg EQ/100 mg) and Tectona grandis (6.79 ± 0.22 mg EQ/100 mg) with the lowest content. In contrast to the findings of Degbe et al., [23] our study showed high levels of flavonoid content in the extracts of Daniellia oliveri (79.81 ± 1.28 mg EQ/100 mg) and Tectona grandis (124 ± 49 mg EQ/100 mg). These differences could be attributed to the specific parts of D. oliveri used and the geographical areas where T. grandis was harvested.
DPPH radical scavenging activity
The results of the DPPH radical scavenging activity and Inhibitory Concentration (IC50) are presented in Figure 1 and Table 2. The analysis of these results indicates that the Tectona grandis extract exhibited the strongest anti-radical power, with an IC50 value of 4.40. It was followed by Pouteria alnifolia (IC50 5.57), Daniellia oliveri (IC50 5.85) and Thalia geniculata (IC50 7.87). Among the four plant extracts, only the antioxidant activity of Tectona grandis extract was comparable to that of ascorbic acid (IC50 3.83). These results demonstrate an inverse relationship between the phenolic compound content in different plant leaf extracts and their antioxidant activity. Notably, the Tectona grandis extract, which has a low phenolic compound content, exhibits strong antioxidant activity. The Pouteria alnifolia extract, with moderately low levels of total flavonoids and high levels of total polyphenols, displays moderate antioxidant activity. Similarly, the Daniellia oliveri extract, which has a low phenolic compound content, shows comparable antioxidant activity to the Pouteria alnifolia extract. On the other hand, the Thalia geniculata extract, with high levels of total flavonoids and moderately high levels of total polyphenols, demonstrates a low ability to reduce free radicals.
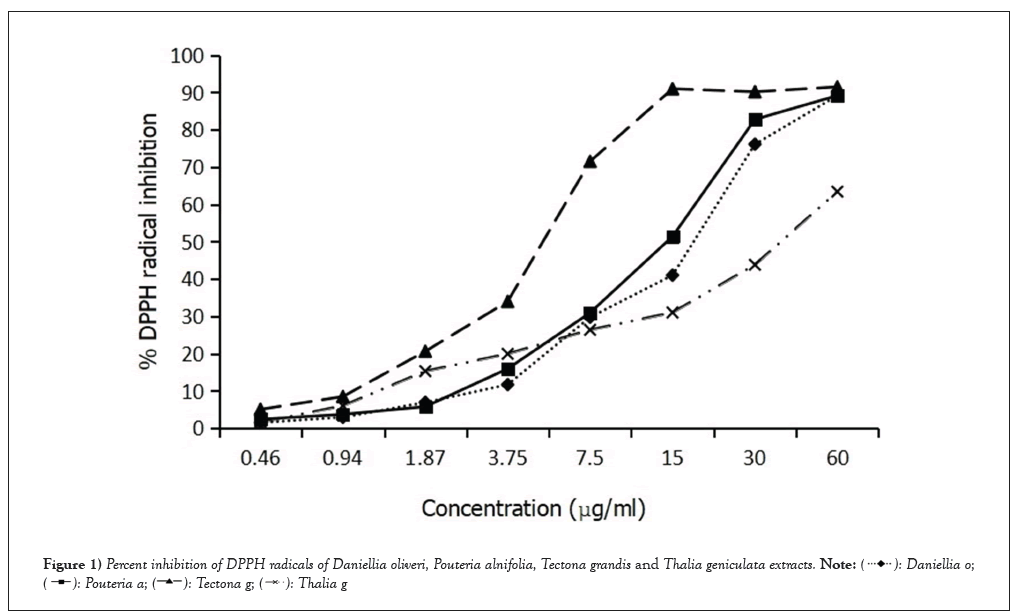
Figure 1: Percent inhibition of DPPH radicals of Daniellia oliveri, Pouteria alnifolia, Tectona grandis and Thalia geniculata extracts. 
 .
.
Plant leaf extracts containing phenolic compounds with a high proton transfer capacity exhibit powerful antioxidant activity, while those with phenolic compounds with low hydrogen donor capacity have lower antioxidant activity. This is because the antioxidant activity of phenolic compounds relies on the mobility of hydrogen atoms in the hydroxyl group.
According to the same author, hydrogen atom donor antioxidants possess the ability to capture free radicals. Additionally, Brand-Williams et al., [24] demonstrated that phenolic compounds with hydroxyl groups in the ortho and para positions exhibit more effective anti-radical activity compared to those with hydroxyl groups in the meta position. Almela et al., [25] further stated that the efficacy of anti-radical activity is influenced by various factors, including the concentration of phenolic compounds, isomeric forms and synergistic interactions with other components. Based on these previous studies, it can be inferred that the observed anti-free radical activity in the leaf extract of the four plants under investigation can be attributed to the nature or structure of the phenolic compounds present in these extracts, rather than the quantity of phenolic compounds.
Antibacterial activity of the extracts
The evaluation of antimicrobial activity involved determining the Minimum Inhibitory Concentration (MIC) of various leaf extracts on Escherichia coli CIP 53126 and Staphylococcus aureus ATCC 6538 strains, as shown in Figure 2. Previous studies have reported the presence of these strains in tomatoes [26-28]. Analysis of the results reveals that the MICs obtained vary depending on the strains and the type of plant extract. Notably, S. aureus exhibited the highest sensitivity, with MIC values ranging from 1.25 to 2.5 mg/mL for the four plant extracts. The extracts of Daniellia oliveri and Tectona grandis were particularly effective against this strain, with an MIC of 1.25 mg/mL. On the other hand, the plant extracts showed lower activity against E. coli, with MIC values ranging from 2.5 to 5 mg/mL. However, E. coli was more sensitive to the extracts of Daniellia oliveri and Thalia geniculata, with an MIC of 2.5 mg/mL. The observed antibacterial activity against the microbial strains may be attributed to the presence of flavonoids, tannins, triterpenes, naphthoquinones and coumarin in the leaf extracts of the plants used. These secondary metabolite families encompass a wide range of biologically active compounds, including antimicrobial properties [29,30]. The MIC values obtained in this study differ significantly from those reported by Onzo et al., [15].
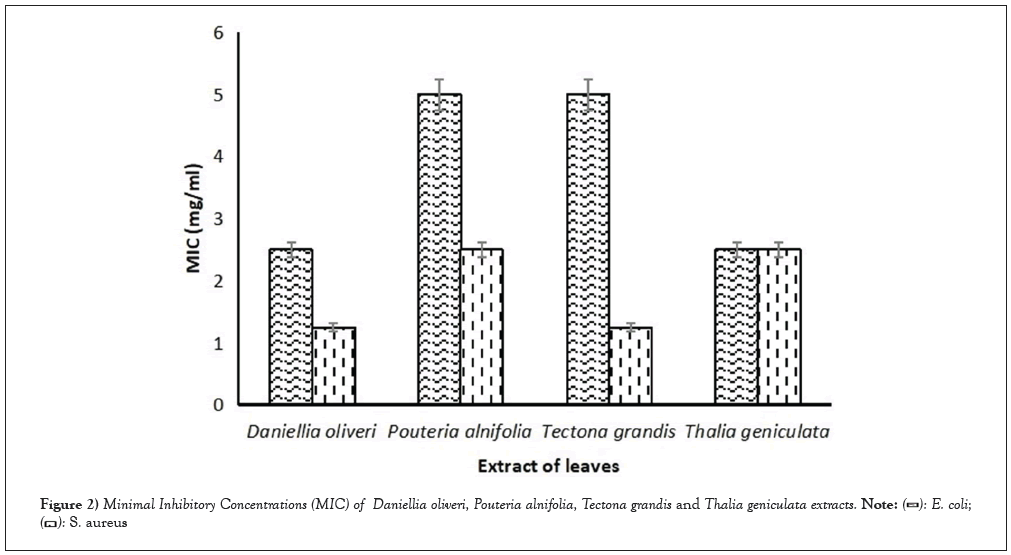
Figure 2: Minimal Inhibitory Concentrations (MIC) of Daniellia oliveri, Pouteria alnifolia, Tectona grandis and Thalia geniculata extracts. 
 .
.
The latter study showed high MIC values of 100 mg/mL for T. geniculata hydroethanol extract (S. aureus) and 1.56 mg/mL (S. aureus) and 3.12 mg/mL (E. coli) for D. oliveri ethanol extract. This discrepancy could be attributed to differences in the extraction solvent, the location of harvest or the maturity state of the leaves. Another study by Anyam [20] reported lower MIC values of 0.62 mg/mL for ethyl acetate extract from Pouteria alnifolia roots against S. aureus and E. coli strains. This finding suggests that the Pouteria alnifolia extract is highly active against both microbial strains, contrary to our results. This discrepancy may be due to differences in the extraction solvent or the plant organ used.
pH variation of tomato puree during storage
The impact of the extracts on the pH changes in tomato puree during a five-day storage period is presented in Table 3. The results indicate that the pH decreased over time for all samples. Notably, the control sample exhibited the highest pH variation (0.21 ± 0.06) between days 0 and 5, while the samples treated with extracts showed a lower pH variation of 0.14 ± 0.05; 0.14 ± 0.03; 0.12 ± 0.06 and 0.14 ± 0.07 for Daniellia oliveri, Pouteria alnifolia, Tectona grandis and Thalia geniculata, respectively. This suggests that all samples containing the extract slowed down the pH decrease compared to the control. It is worth mentioning that the Tectona grandis extract exhibited the smallest pH variation (0.12 ± 0.06). The increase in sample acidity during storage could be attributed to deterioration and fermentation processes, leading to the conversion of sugars into acids, carbon dioxide or alcohol [31]. Microbial growth consumes carbohydrates during fermentation, resulting in lactic acid production and sugar hydrolysis, which contribute to the pH decrease. The significant pH variation observed in the control sample indicates a more pronounced fermentation or deterioration compared to the samples treated with plant extracts.
| Samples | Control | Daniellia oliveri | Pouteria alnifolia | Tectona grandis | Thalia geniculata |
|---|---|---|---|---|---|
| pH | |||||
| Day 0 | 4.26 ± 0.08b | 4.20 ± 0.03b | 4.06 ± 0.09a | 4.08 ± 0.03a | 4.13 ± 0.05b |
| Day 1 | 3.83 ± 0.05b | 3.87 ± 0.03b | 3.76 ± 0.07a | 3.99 ± 0.03c | 3.90 ± 0.02b |
| Day 2 | 3.73 ± 0.02b | 3.67 ± 0.07b | 3.51 ± 0.08a | 3.7 ± 0.07b | 3.63 ± 0.10b |
| Day 3 | 3.56 ± 0.07b | 3.73 ± 0.06c | 3.44 ± 0.03a | 3.66 ± 0.01c | 3.53 ± 0.04b |
| Day 4 | 3.50 ± 0.09a | 3.49 ± 0.10a | 3.43 ± 0.02a | 3.53 ± 0.04b | 3.47 ± 0.09a |
| Day 5 | 3.21 ± 0.03a | 3.49 ± 0.06c | 3.36 ± 0.04b | 3.46 ± 0.06c | 3.43 ± 0.05c |
| ΔpH | 0.21 ± 0.06c | 0.14 ± 0.05b | 0.14 ± 0.03b | 0.12 ± 0.06a | 0.14 ± 0.07b |
| PPO (U/min/g) | |||||
| Day 0 | 78 ± 1.06a | 210 ± 4.11d | 173 ± 1.11c | 268 ± 3.00e | 149 ± 1.29b |
| Day 1 | 75 ± 3.12a | 205 ± 2.37e | 87 ± 3.05b | 188 ± 2.23d | 131 ± 3.25c |
| Day 2 | 54 ± 0.19a | 140 ± 1.29d | 83 ± 3.13b | 152 ± 1.99e | 110 ± 1.97c |
| Day 3 | 39 ± 3.36a | 112 ± 2.09c | 81 ± 1.14b | 125 ± 2.32d | 81 ± 2.32b |
| Day 4 | 20 ± 1.46a | 92 ± 1.176c | 70 ± 2.54b | 121 ± 2.69d | 72 ± 1.45b |
| Day 5 | 18 ± 1.53a | 87 ± 4.01c | 42 ± 1.96b | 118 ± 1.76d | 48 ± 1.38b |
| Δppo | 12 ± 2.46a | 24.6 ± 2.71bc | 26.2 ± 2.34c | 30 ± 1.98d | 20.2 ± 1.73b |
Note: Values are means ± standard deviation (n=3). Data in same line with different letters are significantly different (p<0.05).
Table 3: pH and Polyphenoloxidase (PPO) activity in tomato puree during storage.
Variation in polyphenoloxidase activities of tomato puree during storage
The polyphenoloxidase activities of various tomato puree samples are presented in Table 3. Upon analyzing these results, it is observed that on day 0, the PPO activity of the treated samples is higher than that of the control. This increase could be attributed to a reaction between the phenolic compounds present in the plant extract and the PPO released by the tomato cells during milling in the presence of oxygen. Furthermore, the difference in Δppo activity, which corresponds to the enzymatic inhibition of the samples, between days 0 and 5 of storage is greater in the treated samples compared to the control. However, the different samples containing the extract exhibit a delay in PPO activity compared to the control during 0 to 5 days of storage. Among the samples, Tectona grandis (Δppo=30 ± 1.98) exhibits the highest inhibitory activity in tomato puree during storage, followed by Pouteria alnifolia (Δppo=26.2 ± 2.34), Daniellia oliveri (Δppo=24.6 ± 2.71) and Thalia geniculata (Δppo=20.2±1.73).
These enzyme inhibition results are consistent with the observed antioxidant activity (IC50) for each plant studied. Therefore, it can be inferred that the inhibition of PPO action in the samples is due to the antioxidant activity of the plant extract present in each sample. Soysal [32] discovered that green tea extract, rich in flavonoids, inhibited apple PPO activity. Flavonoids, in addition to acting as reducing agents, inhibit tyrosinase by chelating copper through their free 3-hydroxy group [33]. Secondary metabolites such as tannins in plants form complexes with heavy metal ions like copper [34]. As copper is present in the active PPO site [35], it can be deactivated through its reaction with tannins. Therefore, the inhibition of PPO action by phenolic compounds in the presence of oxygen in tomato puree would slow down the formation of O-quinones and prevent the formation of brown or blackish pigments. This would ultimately extend the shelf life of tomato puree.
Color change during the storage of tomato puree
The analysis of Table 4 reveals that the control sample had higher L*, a* and b* parameters compared to the other samples on day 0. The decrease in these parameters in the treated samples can be attributed to the dark color of the extracts added to the tomato puree. During storage, there was a gradual increase in the L* parameter and a decrease in the a* and b* parameters for all samples. These changes may be due to the degradation of certain secondary metabolites, such as lycopenes and flavonoids, present in the tomato puree. Lycopene, responsible for the red color of tomatoes, can degrade when exposed to oxygen, leading to significant color loss in the puree. The control sample exhibited a greater color variation (ΔE) between days 0 and 5 compared to the treated samples. Among the treated samples, the one containing the extract of Daniellia oliveri showed the lowest color variation, followed by Pouteria alnifolia, Thalia geniculata and Tectona grandis. These differences observed between the treated samples and the control can be attributed to certain phenolic compounds in the extracts that act as substrate analogs, binding to the active sites of PPOs instead of the true substrate. This binding affinity slows down or inhibits the formation of quinones, thus delaying the appearance of the brown or dark color in the tomato puree. According to Wessels et al., [2], phenolic compounds can directly influence the activity of PPO by acting as competitive or non-competitive inhibitors.
| Parametres | Control | Daniellia oliveri | Pouteria alnifolia | Tectona grandis | Thalia geniculata | |
|---|---|---|---|---|---|---|
| Day 0 | L* | 38.75 ± 0.94b | 36.05 ± 1.63a | 34.65 ± 2.01a | 34.76 ± 1.45a | 34.64 ± 1.87a |
| a* | 32.80 ± 1.23c | 14.05 ± 1.53a | 12.02 ± 1.00a | 21.88 ± 1.93b | 13.08 ± 1.33a | |
| b* | 18.85 ± 0.73c | 10.91 ± 1.11a | 9.76 ± 1.34a | 15.31 ± 1.57b | 14.52 ± 1.87b | |
| Day 1 | L* | 39.08 ± 1.82b | 36.46 ± 1.02a | 34.88 ± 1.83a | 34.97 ± 1.78a | 34.86 ± 1.11a |
| a* | 32.58 ± 1.92d | 13.15 ± 1.37a | 11.99 ± 1.56a | 21.68 ± 1.12c | 17.68 ± 1.67b | |
| b* | 17.59 ± 1.47c | 9.56 ± 1.66a | 9.67 ± 1.02a | 14.48 ± 1.92b | 8.65 ± 1.34a | |
| Day 2 | L* | 39.54 ± 0.92b | 36.81 ± 1.83a | 34.93 ± 1.73a | 35.46 ± 1.75a | 35.21 ± 1.41a |
| a* | 32.00 ± 1.03c | 12.83 ± 1.22a | 8.06 ± 1.92a | 21.22 ± 1.66b | 11.72 ± 1.03a | |
| b* | 14.80 ± 1.37c | 9.20 ± 1.73a | 6.35 ± 2.01a | 12.22 ± 1.34b | 8.54 ± 1.56a | |
| Day 3 | L* | 40.41 ± 1.42b | 37.25 ± 1.91a | 35.01 ± 1.42a | 36.81 ± 1.85a | 35.31 ± 1.91a |
| a* | 27.90 ± 1.43d | 12.48 ± 1.39b | 7.52 ± 1.23a | 18.77 ± 1.46c | 11.25 ± 1.43b | |
| b* | 14.02 ± 2.01b | 9.14 ± 2.05a | 6.12 ± 1.63a | 11.33 ± 1.83b | 7.94 ± 1.11a | |
| Day 4 | L* | 41.73 ± 1.06b | 37.92 ± 1.81a | 36.99 ± 1.15a | 38.03 ± 1.09a | 36.02 ± 1.43a |
| a* | 24.48 ± 1.99c | 11.40 ± 0.96b | 7.18 ± 1.35a | 15.35 ± 1.56b | 11.07 ± 1.21b | |
| b* | 13.80 ± 1.73b | 8.82 ± 1.62a | 5.18 ± 1.93a | 9.96 ± 1.22a | 7.26 ± 1.85a | |
| Day 5 | L* | 43.42 ± 1.24b | 38.25 ± 1.37a | 37.04 ± 1.23a | 38.39 ± 1.56a | 39.60 ± 1.32a |
| a* | 22.81 ± 1.83c | 10.11 ± 1.73a | 7.13 ± 1.94a | 14.95 ± 1.99b | 10.27 ± 1.56a | |
| b* | 13.71 ± 1.83b | 6.98 ± 2.22a | 5.10 ± 1.02a | 7.80 ± 1.03a | 7.07 ± 2.02a | |
| ΔE | 12.16 ± 1.39c | 5.98 ± 1.82a | 7.17 ± 1.25a | 10.85 ± 1.71b | 9.38 ± 1.21b | |
Note: Values are means ± standard deviation (n=3). Data in same line with different letters are significantly different (p<0.05).
Table 4: Color change of tomato puree during storage.
Microbiological analysis of tomato puree
The results of the Total Mesophilic Aerobic Flora (TMAF) and the mycelial development of molds and yeasts assessment in tomato puree during storage are presented in Table 5. Upon analyzing the table, it is evident that all samples containing plant extracts exhibited a slower bacterial multiplication compared to the control during storage. Among the extracts, Tectona grandis displayed the highest bacterial inhibitory activity on the fifth day of storage, followed by Daniellia oliveri, Pouteria alnifolia and Thalia geniculata. The lower microbial load observed in the Tectona grandis sample can be attributed to its potent antioxidant activity, which is also correlated with the minimal pH variation observed in this sample during storage.
| Samples | Day 1 | Day 5 | |
|---|---|---|---|
| TAMF (cfu/g) | TAMF (cfu/g) | Mycelium growth (mm) | |
| Control | 71 | ND | 2.7 |
| Daniellia oliveri | 4.15 | 6.41 | 1.5 |
| Pouteria alnifolia | 5.05 | 10.3 | 1.16 |
| Tectona grandis | 3.15 | 3.7 | 1.35 |
| Thalia géniculata | 13 | 14.32 | 1.45 |
Note: TAMF: Total Aerobic Mesophilic Flora; ND: Not Determined; CFU: Colony Size Unit; mm: Millimeters.
Table 5: Total aerobic mesophilic flora and mycelial development in tomato puree during storage.
Regarding the mycelial development of molds and yeasts, all samples containing extracts demonstrated inhibition of these microorganisms in the tomato puree compared to the control during storage. Similar to the TMAF results, the Tectona grandis sample exhibited the highest inhibitory activity on mycelial development, followed by Daniellia oliveri, Pouteria alnifolia and Thalia geniculata throughout the five days of storage. The variation in antimicrobial activity among the different extracts could be attributed to the antioxidant activity of each plant and the structure of the bacterial membrane wall present in the tomato puree. It is worth noting that phenolic compounds, such as flavonoids and tannins, found in various plant extracts, have been reported to possess antimicrobial properties [36].
This study found that the hydrolethanolic extracts of the four leaves primarily consist of secondary metabolites such as coumarins, flavonoids, pigments, saponins, tannins, lignans, triterpenes, naphthoquinones and anthracene derivatives. The content of phenolic compounds varies among the different plant species. Interestingly, the extract from Tectona grandis leaves, which had the lowest phenolic compound content, exhibited a strong antioxidant power compared to the other plant extracts studied. This could be attributed to the nature or structure of these phenolic compounds. Additionally, the hydroethanolic extracts of the four leaves showed more effective antibacterial activity against the S. aureus strain than the E. coli strain. The physicochemical analysis of the tomato puree revealed a slight variation in pH for all the samples containing the extracts of the different plants during storage from day 0 to day 5. This pH variation can be attributed to the low microbial load and reduced growth of yeasts and molds observed during storage. The antioxidant activity of the plant extracts in the samples may contribute to these results. Furthermore, the plant extracts in the samples appeared to have anti-browning properties, inhibiting the activity of polyphenoloxidases in the tomato paste during storage. As a result, the loss of red color in the tomato paste was slowed down in all the samples compared to the control. It is important to note that the hydroethanolic extracts of the four leaves studied had a significant effect on extending the storage period of tomato puree.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Lagnika C, Chanhoun SHS, Amoussa AMO, et al. Antimicrobial activity and enzymatic inhibition of four leaf extracts used as food packaging in the conservation of tomato puree. AGBIR.2024;40(3):1064-1071.
Received: 15-Apr-2024, Manuscript No. AGBIR-24-132241; , Pre QC No. AGBIR-24-132241 (PQ); Editor assigned: 17-Apr-2024, Pre QC No. AGBIR-24-132241 (PQ); Reviewed: 01-May-2024, QC No. AGBIR-24-132241; Revised: 08-May-2024, Manuscript No. AGBIR-24-132241 (R); Published: 15-May-2024, DOI: 10.35248/0970-1907.24.40.1064-1071
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
