Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2023) Volume 39, Issue 2
Tannery industries use a numerous amount of heavy metals in different processes and significant losses during the process and are discharged in the effluent. The present study deals with the analysis of physicochemical parameters of both untreated and treated tannery effluent in Dindigul district. The parameters such as pH, Total Dissolved Solids, Total Alkalinity, Total Hardness, Magnesium, Nitrate etc., and heavy metals like Copper, Iron, Chromium, Zinc, and Lead were analysed using standard protocols. The effluent from the tannery industry was the major source of pollution which will affect the flora and fauna existing in the environment. Biosorption efficiency of coir pith, a waste product from coir industry, was investigated in this study for the removal of heavy metal from tannery effluent. All the parameters were more or less higher for untreated effluent compared to that of the treated. The presence of heavy metal such as Chromium, Copper, Zinc, Iron, and Lead was observed higher in control. After the treatment of effluent with coir pith the heavy metals were reduced to 25% concentration effluent. The present study explores the effectiveness of utilization of coir pith, as a bio sorbent for heavy metal removal. Bioremediation technologies developed can act as good sustainable options for the future in heavy metal removal from industrial effluents.
Tannery effluent; Biosorption; Physico-chemical parameters; Heavy metal; Coir pith; Bioremediation
The tannery effluent stands as highest pollution producer among all other industrial wastes [1]. Tanning industry is one of the oldest and fast growing industries worldwide. India is one of the leading manufacturer of leather which holds third position globally by running 3,000 tanneries with annual processing capacity of 0.7 million tonnes of hides and skin to indigenous use and export [2,3].
The effluent from vegetable tanning process imparts colour and comprise of a non-biodegradable pollutant like tannin which persist for long [4]. Exclusive research works were carried out in recent years, which allow the industrial, municipal, agricultural and commercial facilities to reduce their impact on environment. Particularly, execution of rigorous standard for the release of wastes into the environment had imposed the need for the development of alternative process for the production of goods and for the treatment and clearance of waste [5]. Physical and chemical methods for the treatment of effluent are consistently cost intensive and cannot be applied in all industries particularly in developing and under developed countries. Bioremediation of tannery effluents is an attractive eco-friendly, safe and cost effective alternative technology to the conventional treatment methods [6].
Biometerial
Coconut (Coccus nucifera L.) is one of the most useful extensively cultivated palms in tropical countries such as India. The waste products of coir yarn industry are coir dust and coir pith or coco peat which constitutes about 70% of the husk .The quantity of coir dust produced is so enormous making its disposal difficult because of its lignocellulose nature and slow degradation in the natural environment. Biological treatment of tannery effluent process is carried out to overcome environmental pollution. Coir pith in the tannery effluent would be an ideal absorbent/adsorbent to remove heavy metals and other minerals (Figure 1).
Figure 1: The study area of Dindigul is located in the southern part of Tamil Nadu in India.
Adsorption
Adsorption is one of the promising methods for heavy metal removal and has been proved to be an excellent method to treat industrial waste effluents offering significant advantages like their low cost, easy availability, profitability, ease of operation and efficiency. Adsorption is a mass transfer process by which a substance is transferred between any two phases, such as liquid–liquid, gas–liquid, gas–solid or liquid–solid interfaces [7,8]. Several studies have been done by researchers in exploring the effectiveness of different materials as adsorbents. Commercial Activated Carbon (CAC) is widely used as an adsorbent for removing different types of pollutants from wastewater but is an expensive material and hence adsorption systems based on it are not economically viable [9]. Bio sorption using low-cost adsorbents for removing metallic pollutants is a comparatively new technology (only in the 1990s) and has a high potential for being developed as a cost effective one [10].
Study area
Dindigul is one of the major industrial and commercial towns of Tamil Nadu.
Collection of tannery effluent
The effluent was collected from outlet of tannery effluent located Dindigul district. Samples were collected in wide mouthed plastic bottles. The plastic bottles were properly washed with detergent and distilled water prior to water collection and were carefully rinsed with sample effluent filled up to the brim and tight closed to ensure bubble free sample storage [11].
Bio adsorbent preparation
The coconut coir pith used in these studies for the removal of heavy metals from tannery effluent. Raw pith was collected from a nearby industry. It was washed several times with distilled water to remove soil and dust particles. It was sundried by exposure to the sun light for 3 days; the dried material was ground in to a powder using blender and sieved to obtain constant finer size particles. Characterization of pith was done to study the parameters such as surface area, lignin content, etc. The adsorption rate is strongly influenced by several parameters such as pH, contact time, initial concentration, adsorbent dosage, adsorbent size, etc. These parameters are optimized for getting maximum removal efficiency for heavy metals.
Preparation of water sample for absorption
The tannery effluent was diluted with distilled water. Each concentration contained 100%, 75%, 50%, and 25% of tannery effluent.
• 100% effluent
• 75% effluent+25% distilled water
• 50% effluent+ 50% distilled water
• 25% effluent+75% distilled water
Introducing adsorbent in effluent
500 g of adsorbent was introduced in each concentrated effluent. The effluent was soaked till the experiment work was finished.
Characterization of coir pith parameters
The physical and chemical properties of an adsorbent play an important role in reduction of heavy metal. Characterization studies on coir pith are shown in Table 1.
| Parameters value | P value meters |
|---|---|
| Lignin (%) | 29.6 |
| Hollocellulose (%) | 42.3 |
| Bulk density (g/cc) | 0.097 |
| Particle density (g/cc) | 0.985 |
| Pore space volume (%) | 88.5 |
| Particle size (micron) | 344 |
Table 1: Characteristics of coir pith.
It can be seen that coir pith is having a high percentage composition of lignin (29.6%) and hollocellulose (42.3%) which are responsible for its physical stability causing poor biodegradability. These characteristics are typical for agricultural by products and are referred to as lignocellulosic materials. Lignin and cellulose are biopolymers bearing multiple phenolic, hydroxyl, carboxyl and amino groups which are reported as responsible for the removal of pollutants from waste water. The low values of bulk density and high values of pore space volume of coir pith are found to be favourable for the process of adsorption.
In the present investigation, tannery effluent was collected from a common treatment effluent plant in the period of study. The physicochemical parameters of the effluents were analysed (Table 2). The result of the study revealed that colour of the untreated effluent was greenish in colour with offensive odour. A large number of pollutants can impart colour, taste and odour to the receiving water, thereby making them anaesthetic and unfit for domestic consumption [12]. However in the present study, treated effluent was light yellow, the odour was objectionable to that of the untreated. Brown colour of the tannery effluent was also reported by Smrithi, et al [13]. The colour of the effluent might be due to the presence of biodegradable and non-biodegradable high molecular weight organic compounds and high amount of inorganic chemicals like sodium and chromium used during the processing and the odour may be due to putrefaction of the organic residues from the processed skin and hides [13].
| Parameters | Permissible value in normal water | Untreated | Treated |
|---|---|---|---|
| Colour | - | Greenish | Light yellow |
| Odour | - | Objectionable | Unobjectionable |
| Turbidity | 10 | 30 | 16 |
| Total dissoloved solids (mg/l) | 20 | 80 | 45 |
| Electrical conductivity | - | 8070 | 6560 |
| pH | 6.5 | 8.8 | 6.2 |
| Total alkalinity (mg/l) | - | 988 | 472 |
| Total hardness (mg/l) | 200 | 788 | 412 |
| Magnesium | 30-100 | 370 | 257 |
| Nitrate (mg/l) | 45 | 70 | 52 |
Table 2: Physicochemical parameters of untreated and treated tannery effluent in Dindigul district.
Total dissolved solids are mainly due to carbonates, bicarbonates, chlorides, sulphates, phosphates, nitrates, nitrogen, calcium, sodium, potassium and iron [14]. The presence of high level of TSS and TDS may be due to the insoluble organic and inorganic present in the effluent [15]. In our study untreated effluent showed higher level of electrical conductivity, 8070 which indicates that the discharge of chemicals as cations and anions were higher in the waste water. The higher conductivity alters the chelating properties of water bodies and creates an imbalance of free metal availability for flora and fauna [16]. It may be due to high concentration of acid base and salt in the effluent [12]. However, the electrical conductivity of the treated effluent was also higher, 6560. The average pH of the untreated tannery effluent was found to be within the range 8.8 similar to that of Rabah and Ibrahim [17]. This could explain the high counts of microorganisms because most of them thrive well in such pH value. Total alkalinity is higher in untreated effluent 988 mg/L than the treated effluent 472 mg/L alkalinity of water is its acid neutralizing capacity. It is the sum of all the bases. The alkalinity of natural water is due to the salt of carbonates, bicarbonates, borates, silicates and phosphates along with hydroxyl ions in the Free State (Table 3).
| Heavy metal | Concentration of tannery effluent | Untreated | Treated | Absorption |
|---|---|---|---|---|
| Zinc | 100% | 0.7 | 0.675 | 0.025 |
| 75% | 0.6 | 0.582 | 0.018 | |
| 50% | 0.5 | 0.491 | 0.009 | |
| 25% | 0.3 | 0.297 | 0.003 | |
| Iron | 100% | 2.4 | 2.311 | 0.089 |
| 75% | 2.0 | 1.925 | 0.075 | |
| 50% | 1.2 | 1.145 | 0.055 | |
| 25% | 1.0 | 0.955 | 0.045 | |
| Chromium | 100% | 9.5 | 9.413 | 0.087 |
| 75% | 9.0 | 8.923 | 0.077 | |
| 50% | 8.0 | 7.944 | 0.056 | |
| 25% | 6.0 | 5.952 | 0.048 | |
| Copper | 100% | 0.8 | 0.752 | 0.048 |
| 75% | 0.7 | 0.662 | 0.038 | |
| 50% | 0.5 | 0.481 | 0.019 | |
| 25% | 0.2 | 0.192 | 0.008 | |
| Lead | 100% | 6.5 | 6.412 | 0.088 |
| 75% | 5.0 | 4.921 | 0.079 | |
| 50% | 4.0 | 3.933 | 0.067 | |
| 25% | 3.0 | 2.951 | 0.049 |
Table 3: Absorption of different heavy metals in various concentrations by using coir pith.
In the present investigation, total hardness of untreated effluent was found to be high, 788 mg/L than the permissible limit of CPCB (1995), whereas for treated effluent, the values were 412 mg/L similarly, total hardness 1416-3850 mg/L was observed for untreated effluent [6]. Calcium, magnesium, carbonates, bicarbonates, sulphates, chlorides, nitrates, organic matter together associate and form hardness of water. Magnesium content 370 mg/L was found to be maximum in the untreated effluent. In the present study, the nitrate values of untreated effluent were 70 mg/L whereas for treated effluent, the values were 52 mg/L.
The physicochemical properties and heavy metals concentration of the effluent varies depending on the process of tanning adopted in various industries [18]. The tannery waste water is being contaminated with higher levels of metals (iron, nickel, chromium, zinc, cadmium, manganese and copper) and these metals contaminate the agricultural soil. The crops and vegetables, which when consumed cause serious health hazards to the consumer [14]. Higher value of chromium (9.5 mg/L) was found in the untreated effluent during the period whereas, higher value of copper (0.8 mg/L) was found in the untreated effluent during the period. Maximum level of zinc (0.7 mg/L), Iron (mg/L) 2.4 and Lead (6.5 mg/L) in untreated effluent was reported in the present study (Figure 2). Continuous discharge of chromium in low concentration has been reported to be toxic to aquatic life and has been shown to disrupt the aquatic food chain [19]. Copper is an essential element in mammalian nutrition as a component of metalloenzymes in which it acts as an electron donor or acceptor. Conversely, exposure to high levels of copper can result in a number of adverse health effects [20]. Acute toxicity of zinc may result in sweet taste, throat dryness, cough, weakness, generalized aching, chills, fever, nausea and vomiting [21]. Thus the analysis of physicochemical parameters of untreated effluent for the period, confirms that the waste water released from the tannery industry has higher concentration of Turbidity, TDS, EC, pH, Total alkalinity, Total Hardness, Magnesium, Nitrate, etc. which exceeded the permissible limits prescribed for discharge of industrial effluent into inland surface water as well as on land for irrigation than the treated effluent. From the result of physicochemical analysis of untreated and treated tannery effluents, it has been concluded that of Turbidity, TDS, EC, pH, Total alkalinity, Total Hardness, Magnesium, Nitrate, etc. are very high in concentration compared to the treated effluent. Heavy metal concentration also shows great variability. They are unfit for irrigation. The effluents containing noxious pollutants lead to environmental problems which will affect plant, animal and even human life [22].
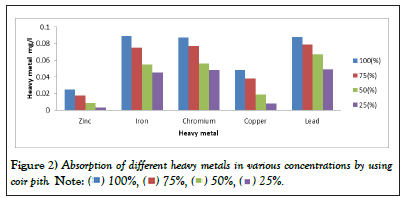
Figure 2: Absorption of different heavy metals in various concentrations by using
coir pith. 
Tannery industries produce large amounts of waste water and release directly to the water bodies near to industries thus polluting the environment. Tannery effluent treatment before discharge is important to reduce the environmental damage. In the present study, the performance of tannery effluent treatment and removal of heavy metals and physiochemical parameters were observed. Before treatment the effluent from tannery industry had high value of Total hardness, Turbidity and Electrical conductivity and it was reduced to considerably after treating it with coir pith. The chemical parameters such as pH, Magnesium and Nitrate etc., were higher in control effluent. After treating it with coir pith the chemical parameter of tannery effluent reduced significantly. The presence of heavy metal such as Chromium, Copper, Zinc, Iron, and Lead was observed higher in control. After the treatment of effluent with coir pith the heavy metals were reduced to 25% concentration effluent. It can be concluded that coir pith has the potential for commercialization as it is technically feasible, eco-friendly, cheap and having good metal binding capacity. Bio sorption by lowcost adsorbents is considered as a comparatively new technology, and is facing several limitations such as lower efficiency, high operational cost, disposal of spent adsorbents, etc. Hence, the above treated tannery effluent can be used as eco-friendly fertilizer and thus leads to soil enrichment, restoring the soil fertility, protecting it against drought, soil disease, stimulate plant growth and plant resistant to unfavourable environmental stresses.
[Crossref] [Google Scholar] [Pub Med]
Citation: Banu JS, Rose DMR. Bioremediation of tannery effluent using bioabsorbent (coir pith) for removal of heavy metal from tanning industry in Dindigul district. AGBIR.2023; 39(2):487-490.
Received: 02-Feb-2023, Manuscript No. AGBIR-23-88513; , Pre QC No. AGBIR-23-88513 (PQ); Editor assigned: 07-Feb-2023, Pre QC No. AGBIR-23-88513 (PQ); Reviewed: 21-Feb-2023, QC No. AGBIR-23-88513; Revised: 28-Feb-2023, Manuscript No. AGBIR-23-88513 (R); Published: 07-Mar-2023, DOI: 10.35248/0970-1907.23.39.487-490
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
