Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2024) Volume 40, Issue 1
The development of biotechnology and agriculture relies heavily on callus culture, an integral part of plant tissue culture, Conserve and improve economically and ecologically significant plant species by advancing our understanding of plant physiology and biochemistry and facilitating the rapid propagation of clones. Plant tissue culture has emerged as a feasible method for producing chemicals and the elicitation methodology can aid in their synthesis. Alpinia purpurata, a member of the Zingiberaceae family, is a useful medicinal herb and having a property against cancer, free radicals, and bacteria are among them.
In this study, A. purpurata in vitro callus cultures were subjected to elicitation treatment for four weeks, during which time the effects of Methyl Jasmonate (MJ) and Salicylic Acid (SA) as elicitors were examined. As a part of the post-elicitation analysis, methanol was extracted from the dried callus. Both the nature of the elicitor and the length of time that it was present had significant effects on the measurable outcomes. Overall, treatment groups showed a considerable improvement in flavonoids synthesis compared to the control group. After 21 days of elicitation with a specific quantity of MJ, the highest levels of three flavonoids were found, as quantified by High Performance Liquid Chromatography (HPLC) analysis. Myricetin (489.90 ± 5.99 µg/g DW), Quercetin (994.22 ± 2.15 µg/g DW) and Kaempferol (120.12 ± 2.12 µg/g DW) concentrations were determined. All three flavonoids exhibited a threefold rise and 100 M in MJ concentration compared to the control group. Myricetin (244.62 ± 3.18 µg/g DW), Quercetin (446.53 ± 2.25 µg/g DW), and Kaempferol (60.80 ± 1.21 µg/g DW) all increased by more than 1.5-fold with 50 µM SA as compared to the control group after induction. Based on this research, we may conclude that MJ is a more potent elicitor than SA at promoting flavonoid production in A. purpurata callus cultures.
Alpinia purpurata; Callus cultures; Flavonoids; Elicitation; Methyl jasmonate; Salicylic acid
Medicinal plants provide the access to potentially life-saving medications for a significant proportion of the global population. In recent decades, there has been an observable increase in the utilization of plant cells for the production of valuable chemicals, either through natural means or via genetic engineering [1]. The Zingiberaceae family of plants has garnered significant attention due to their diverse applications in the field of medical treatment [2-4]. A. purpurata K. Schum, usually referred to as red ginger, is a well favored plant for decorative purposes. The plant is exclusively found in the Asia-Pacific islands. However, its striking red tropical blossoms have gained global recognition [5,6]. The rhizome of the A. purpurata plant offers a number of useful properties, such as a strong fragrance, heightened hunger, improved taste perception, and greater vocal capabilities. Furthermore, it is employed for the purpose of mitigating symptoms associated with rheumatism, sore throat, and kidney disease. Cough can be alleviated by consuming a floral infusion [7,8]. A. purpurata possesses larvicidal, cytotoxic, antibacterial, and vasodilatory qualities [9].
According to Feher [10], in an in vitro setting, a plant cell has the characteristic of totipotency, enabling it to produce many components of the parent plant. Callus formation is observed on recently dissected explants in growth-promoting environments as a result of unregulated growth and cellular proliferation [11,12]. Callus production can be induced from explants derived from varied plant parts [13]. By employing conditions that are supplemented with elevated concentrations of auxin or a combination of auxin and cytokinin [14,15].
Development of callus culture plays a crucial role in the preservation of endangered plant species. It enables the sustainable synthesis of therapeutic chemicals without the need to harvest the entire plant, thereby providing an alternative source [16]. The establishment of callus culture plays a pivotal role in the use of biotechnology for the exploitation of plant-derived resources to serve human interests. This technique enables the manipulation of plant materials to generate valuable products that find applications in various industries such as pharmaceuticals, food, agriculture and cosmetics [17]. In recent years, the utilization of callus culture as a biological engineering methodology for the synthesis of medicinal chemicals, has garnered significant attention. This approach involves applying diverse methodologies to optimize the synthesis of beneficial molecules by targeted plant cells [18]. The current study detected a significant production of secondary metabolites by the callus culture being investigated. Anticancer compounds such as Paclitaxel are produced from the callus culture of Taxus baccata and Camptothecin was extracted from the callus and suspension cultures of Ophiorrhiza mungos L. var. angustifolia (Thw.) [19,20]. Antibiotics from callus culture of Ammi visnaga for antibacterial activity reported by Al-saleh et al., and Begum et al., shows that callus culture for the production of antifungal activity by the extracts of Cichorum intybus to a conventional antifungal Nystatin medication [21,22]. Antivirals and antioxidant compounds are produced from callus culture [15]. Antibodies derived from Dancus carrota callus cultures have also been used to develop a potential oral vaccination against atherosclerosis [23]. Bevacizumab, a monoclonal antibody employed for the treatment of colon cancer, lung cancer, glioblastoma, and renal-cell carcinoma, has been generated through the utilization of transgenic callus cultures derived from rice [24].
A. purpurata uses the phenylpropanoid pathway to convert phenylalanine into flavanols. The flavonols myricetin, quercetin and kaempferol are the most widely distributed and three flavanols are glycosides, conjugated chemicals connected to sugar molecules through glycosidic bond. Myricetin exhibits a diverse range of pharmacological activities, encompassing neuroprotective, antidiabetic, anticancer, immunomodulatory, cardiovascular, analgesic, and antihypertensive actions. In a study conducted by Taheri et al., [25] the flavonoid quercetin possesses numerous therapeutic uses like antioxidant, antibacterial, anti-inflammatory, antiviral, and anticancer properties have found that quercetin exhibits protective effects against neurodegenerative disorders such as alzheimer's, parkinson's, huntington's, and epilepsy [26,27]. Ding et al., [28], revealed quercetin in the context of cigarette smoking-induced Chronic Obstructive Pulmonary Disease (COPD) can be attributed to its immunomodulatory, anti-cellular senescence, mitochondrial autophagy-modulating, and gut microbiota-modulating properties. Xiushen Li et al., [29], stated that quercetin possesses potential therapeutic capabilities for the treatment of Colon Adenocarcinoma (COAD) and COVID-19. Kaempferol inhibits the in vitro replication of varicella-zoster virus. It has been shown that kaempferol inhibits the DNA replication step of the viral life cycle [30]. Antioxidant, anti-inflammatory, and cardio protective effects have been shown [31]. Kaempferol has promising properties for the treatment of atherosclerosis, neuroprotective and ischemic stroke [32]. The immunomodulatory properties of kaempferol and its ability to inhibit tryptophan breakdown were first noted by study of Hofer et al., [33] and its interaction with Indoleamine 2,3-Dioxygenase 1 (IDO-1) is probably responsible for this inhibition.
The biotechnological production of advantageous molecules using elicitation is being investigated. MJ and SA are essential for plant growth and stress tolerance, according to biotechnology. Chemicals stimulate secondary metabolism in plants. MJ, an aromatic and volatile jasmonic acid ester, alters secondary metabolism and triggers plant defenses [34]. According to Rubio-Rodrguez et al., [35], MJ has the potential to be a chemical elicitor. Although SA lowers salinity, drought, cold, and UV radiation, most research has focused on its abiotic and biotic stress tolerance [36]. Reprogramming of the secondary metabolite synthesis pathway takes place when biotic and abiotic chemicals enter plant cells via cell membrane receptors [37]. This, elicitor treatment boosts secondary metabolite synthesis increases Reactive Oxygen Species (ROS) production which in turn regulates gene expression, protein function, and metabolism [38]. Environmental stress increases ROS production, resulting in an oxidative burst. Plant defenses are activated when ROS levels are high. Flavonoids, phenolic acids, and anthocyanins provide this protection [39,40].
The rhizome of A. purpurata is the primary site for the presence of the plant's bioactive compounds. Callus culture was established with the objective of enhancing flavonoid production by elicitation. The objective of this study was to investigate the impact of MJ and SA on the accumulation of flavonoids in callus cultures of A. purpurata. The efficacy of an elicitor is recognized by the duration of exposure of the elicitor.
In vitro establishment
The rhizomes of A. purpurata were acquired from Rajadhani agro farms located in Hyderabad, India, for the purpose of in vitro establishment. The rhizome and its buds were predisposed to an extensive washing process for a duration of 10 minutes using a mild detergent and continuous flow of tap water. Subsequently, the rhizome buds given a 5-minute treatment wherein a Bavistin solution with a concentration of 0.1% w/v was applied. Surface sterilization with 0.1% (w/v) HgCl2 solution was applied to the rhizome buds for a duration of 3 minutes. Subsequently, the buds were subjected to ten rinse cycles using sterile double-distilled water. The use of this rigorous methodology was undertaken to ensure the complete elimination of any residual HgCl2. The rhizomes were dissected to excise the buds, which were subsequently cultured under aseptic conditions of laminar air flow on Murashige and Skoog (MS) semi-solid medium, supplemented with 3% (w/v) sucrose and complemented with (Benzyaminopurine (BAP), kinetin (Kn), Naphthalene Acetic Acid (NAA), Indole-3-Acetic Acid (IAA) and Indole-3-Butyric Acid (IBA)).
In vitro plant propagation
The Biochemical Oxygen Demand (BOD) incubator (Amerex, Lafayette, CA) was utilized to ensure the ideal conditions for the inoculated explants. The experimental parameters consisted of a temperature of 25 ± 2°C, an incident light intensity of 60 Mol/m²/s, and a photoperiod consists of a duration of 16 hours of light, which is then followed by a period of 8 hours of darkness [41].
Callus induction
Diverse explants viz root, rhizomes, and leaves from in vitro grown plants were used for callus induction. The selected explants were excised as thin circular discs and are cultured in petri dishes containing semisolid MS media fortified with different combinations of auxins and cytokinins viz. 2 2,4-D+0.5 Kn+0.5 BAP. The explants were incubated under standard culture conditions. Callus induction can be seen by a small protrusion of cells from the cultured tissue, these were then transferred to a fresh media for further proliferation and were sub-cultured every 28days. Each petri dish housed six explants, and each treatment had at least three duplicates. Following three weeks of culture, each treatments callus induction rate, texture, and colour were recorded for each treatment. The data was then computed using the following formulas.
Callus Induction Rate (%) = Total Number of Cultured Explants/Explants Producing Callus × 100
Callus Browning Rate (%) = Number of Browning Explants/Total Number of Cultured Explants × 100
After three weeks, the original calli were sub cultured on MS basal medium supplemented with the same Plant Growth Regulators (PGRs) as the callus induction media. Several characteristics were measured during subcultures, including biomass, proliferating calli colour, and callus growth index.
Growth kinetics
The aseptically established callus was used for callus proliferation on MS media augmented with 2.0 2,4D mg L-1 +0.5 KN mg L-1 +0.5 BAP mg L-1. The growth studies of callus culture were carried out by recording the growth of the callus for 30 days. The growth experiments were carried out using a standardized combination of 2.0 2,4D mg L-1 +0.5 KN mg L-1 +0.5 BAP mg L-1 in MS media augmented with 3% sucrose. The inoculum density was 15% w/v. The biomass and the secondary metabolite content of the samples was analysed at an interval of 5 days for a duration of 30 days.
Production kinetics
The samples obtained from growth kinetics, with a time interval of 5 days throughout a 30-day period, were subjected to drying and subsequently utilized for the extraction and analysis of flavonoids, specifically Myricetin, Quercetin, and Kaempferol.
Elicitor treatment
The utilization of MJ and SA as signalling molecules was employed as elicitors to examine their influence on the biosynthesis of flavonoids in callus cultures of A. purpurata. The MJ and SA stocks were prepared at a concentration of 1 milliMolar (mM) in a solution of 50% ethanol. The stocks were filter sterilized using a syringe-driven filter (Millipore, Nylon, Lucknow, India) with a pore size of 0.22 µM. The callus cultures were treated with MJ and SA for elicitation. Elicitation studies were conducted with various concentrations of MJ and SA, which were (50 µM, 100 µM, 150 µM, and 200 µM). The callus cultures were harvested at specific time points, on 7, 14, 21, and 28 days following the application of an elicitor. The purpose of this study was to ascertain the ideal period for inducing the most favourable response in terms of flavonoid production.
Sample preparation for Myricetin, Quercetin, and Kaempferol quantification
The callus cultures of A. purpurata were utilized for the purpose of extracting and quantifying the flavonoids. Prior to extraction, the callus samples were subjected to a drying process at room temperature for the duration of 2 days to eliminate excess moisture. 200 mg of dried callus was pulverized using a mortar and pestle by adding 20 ml of methanol to the powder and subjecting it to sonication using a tip sonicater at a temperature of 60°C for a duration of 30 minutes. The purpose of this process was to disrupt the glycosidic bonds that are present in the flavonoids. Subsequently, filtration was performed using a filter with a pore size of 0.22 µm. The filtrate was utilised for analysis using HPLC. The analysis of flavonoids was conducted periodically in conjunction with the development of control callus cultures and elicited callus cultures.
HPLC analysis
HPLC was conducted to quantify the flavonoids present in the elicited and control callus cultures, using the procedure [42]. The HPLC system employed in this investigation was the Shimadzu LC 20AD series HPLC System, which was outfitted with a C18 column containing octadecyl silane and system utilized in this study was the prominence model. During the analysis, the flow rate was adjusted to 1 ml/min, and a 20 µl injection volume was employed. The analytical approach utilized in this study was isocratic. The mobile phase consisted of HPLC grade water containing 0.1% orthophosphoric acid, mixed with acetonitrile in a ratio of 65:35. The mobile phase was filtered to eliminate fine particles, and subsequently sonication for a period of 20 minutes to eliminate any gas bubbles. The authentic samples of Myricetin, Quercetin, and Kaempferol were obtained from Sigma Aldrich, located in Bangalore, India. The standards were dissolved in methanol of high HPLC grade methanol a concentration of 1 mg/ml. The quantification was performed using a UV detector set at a wavelength of 220 nm.
Statistical analysis
The data was gathered in duplicate to ensure accuracy and reliability for subsequent statistical analysis. The resulting measurements were then recorded, including the mean value and Standard Deviation (SD). The data sets were compared using SAS's (Statistical Analysis System, Version 9.1) least significant difference (p0.05) test.
Invitro plant establishment
The establishment of plants through in vitro propagation is a critical method for the successful cultivation of medicinal plants on a wide scale. The rhizome buds of A. purpurata were subjected to surface sterilization, dissection, and subsequent transfer onto MS media. The MS media was supplemented with several concentrations and combinations of phytohormones, viz BAP (0.5 to 3.0 mg L-1), Kn and NAA (at concentrations of 0.1 and 0.5 mg L-1).
The most favorable outcomes in shoot proliferation and shot numbers were achieved when using MS media supplemented with a mixture of 1.5 mg L-1 BAP, 0.5 mg L-1 Kn, and 0.5 mg L-1 NAA. This particular treatment resulted in shoot formation without any accompanying basal callus formation or growth inhibition. A total of 14.0 ± 0.03 shoots were observed on the media combinations after a duration of 4 weeks of culturing. The response of shoot multiplication was not shown to be effective at lower concentrations of BAP and NAA. Nevertheless, the application of a 1.5 mg L-1 concentration of BAP resulted in an increased number of shoots when used in conjunction with Kn and NAA. Shoot proliferation was negatively impacted by higher concentrations of BAP, i.e, 3.0 mg L-1 (Figure 1).
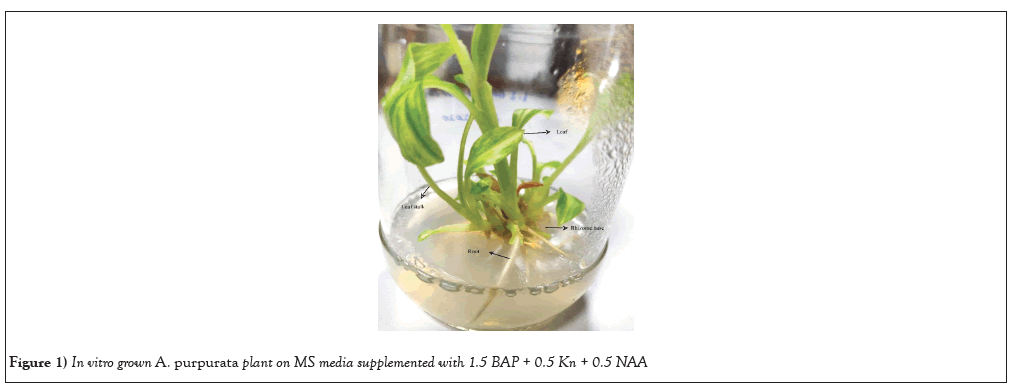
Figure 1: In vitro grown A. purpurata plant on MS media supplemented with 1.5 BAP + 0.5 Kn + 0.5 NAA.
Callus induction
Callus was induced from in vitro produced A. purpurata plants using a variety of explants, including leaves, shoot bases and root sections. Different cytokinin BAP, Kn (0, 0.5, 1, 2 mg/L) and auxin 2,4-D (0.5, 1, 1.5, 2, 2.5 mg/L) concentrations and combinations were tested for their effectiveness in inducing callus formation.
The best response for callus induction was obtained from shoot base explants in the presence of 2ml 2,4-D mg/L, BAP 0.5 mg/L and 0.5 Kn mg/L after 28 days of incubation. The response was about 100%. The other concentrations showed response of about 50-60%. Lower concentration of 2,4D was not effective for callus induction response. However, 2,4-D concentration of 2 mg L-1 enhanced the callus induction with BAP and Kn. Higher concentrations of BAP and Kn showed response of 30-50%. Other explants like leaf section and root sections did not show any callus induction response. Following a four-week period of callus initiation, data pertaining to the rate of callus induction, texture, and color were collected for each treatment using PGRs. These findings are provided in Table 1. Upon conducting a comparison of the concentrations and combinations of cytokinin and auxin, it was noted that the combination of Kin + 2,4-D and BAP resulted in a callus induction stimulation of 100 µ 0.00% when added to the MS medium.
| Medium no | 2,4 D mg/L | BAP mg/L | Kn mg/L | Callus induction% | Browning rate | Texture | Colour |
|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 0.1 | 0.1 | 30 ± 0.05 | 60 | Friable | Brown |
| 2 | 1 | 0.5 | 0.5 | 42 ± 0.05 | 50 | Friable | Brown |
| 3 | 1.5 | 0.5 | 0.5 | 56 ± 0.05 | 40 | Friable | Half white |
| 4 | 2 | 0.5 | 0.5 | 100 ± 0.00 | 10 | Friable | Half white |
| 5 | 2.5 | 1 | 1 | 100 ± 0.00 | 30 | Friable | Half white |
| 6 | 0.1 | 1.5 | 1.5 | 26 ± 0.05 | 70 | Compact | Half white |
Table 1: Callus induction rate, browning rate, colour and texture data of callus on induction media.
Shoot base explants has proven to be the best source for callus induction, because of the protrusion of small compact mass of cells was observed after 28days of culture in petri dishes (Figures 2-4). An undifferentiated and unorganized mass of plant parenchyma cells result in plant callus formation. Earlier report of callus formation was derived from the shoot base of Prunus avium L using 0.5 M3B medium [43]. Callus was induced from the shoot bases of Arachis stenosperma and Arachis villosa [44]. Consequently, the exploration of callus culture derived from shoot base explants has been a fascinating avenue of research, encompassing various plant species.
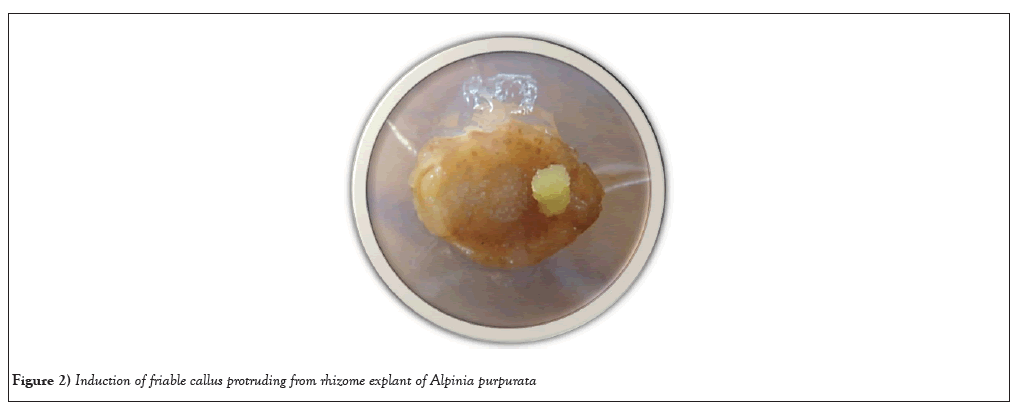
Figure 2: Induction of friable callus protruding from rhizome explant of Alpinia purpurata.
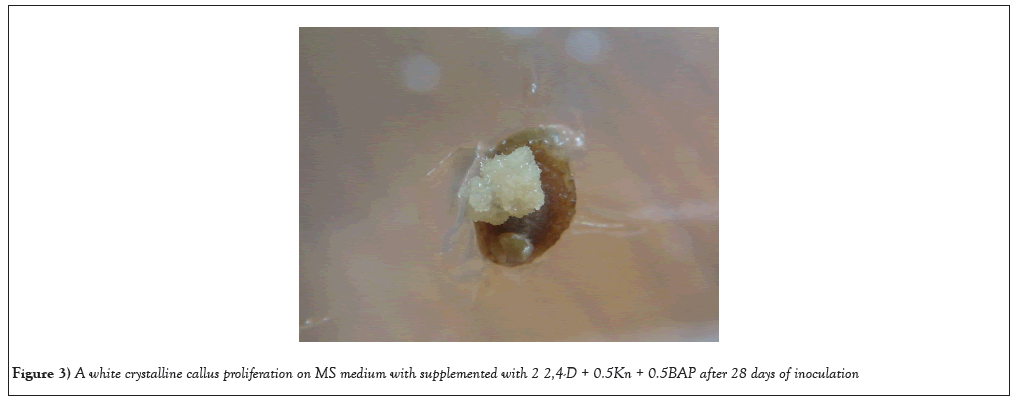
Figure 3: A white crystalline callus proliferation on MS medium with supplemented with 2 2,4-D + 0.5Kn + 0.5BAP after 28 days of inoculation.
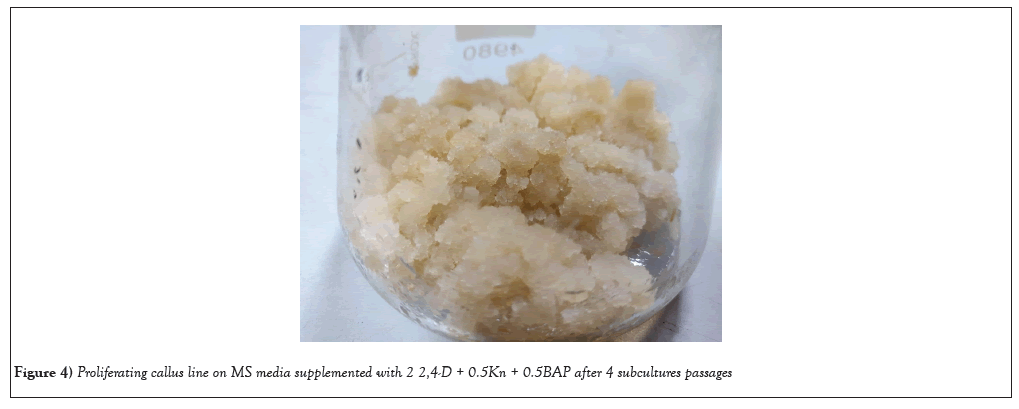
Figure 4: Proliferating callus line on MS media supplemented with 2 2,4-D + 0.5Kn + 0.5BAP after 4 subcultures passages.
Growth studies
The growth studies of the callus cultures were investigated using optimal physical parameters. In this study, the growth medium used was MS medium, which was enriched with 3% sucrose and a combination of 2 mg L-1 2,4 D, 0.5 mg L-1 Kn, and 0.5 mg L-1 BAP. In order to assess biomass, an inoculum density of 15% w/v (4.5 g) was employed as the initial mass, and biomass was quantified in terms of gram Fresh Weight (gFW). The growth phase of cultures exerted a significant influence on both culture morphogenesis and flavonoid production. The relationship between the Growth Index (GI) and flavonoid production, measured in terms of fresh weight (g FW), was examined during a 30-day period, with data collected at 5-day intervals. The callus cultures demonstrated a consistent growth pattern, as indicated by the increase in GI on gFW basis. This growth was seen to be directly proportional to the duration of the culture period. The highest biomass of 7.2 (gFW) was attained on the 20th day of culture. However, subsequent to this peak, the biomass exhibited a fall (Figure 5).
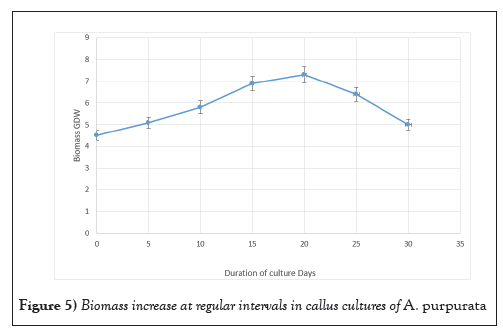
Figure 5: Biomass increase at regular intervals in callus cultures of A. purpurata.
Numerous research investigated the development of callus cultures over time. Most of the growth of B. striata calluses occurred during the stationary period, which occurred on day 36 [45]. Rapid callus growth was seen in Sophora japonica beginning at day 7, with the peak yield occurring at day 35 [46]. Callus development in Coffea canephora also followed a sigmoidal curve, with sub culturing being optimal on day 53 [47]. The callus weight of Azadirachta indica was greatest on day 24 [48] (Figure 6).
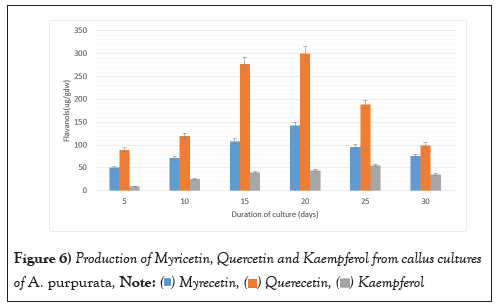
Figure 6: Production of Myricetin, Quercetin and Kaempferol from callus cultures
of A. purpurata.  .
.
The callus cultures were harvested, weighed, and dried at room temperature for 3 days. The extracts were evaluated for the presence of flavonoids viz. Myrecetin, Querecetin and Kaempferol. As displayed in callus cultures displayed maximum content of Quercetin which was 300.26 ± 2.15 µg. gDW-1 followed by myricetin which was 153.31 ± 1.35 µg. gDW-1(Figure 7). Kaempferol was produced to lesser extent in comparison to other two flavonoids which was 55.12 ± 1.16 µg. gDW-1. All the three flavonoids displayed similar pattern for their production which is related to the growth of the callus cultures. The flavonoid peaks were detected in the authentic sample within the flavonoid region, with retention times of 5.2, 8.4, and 14.3.
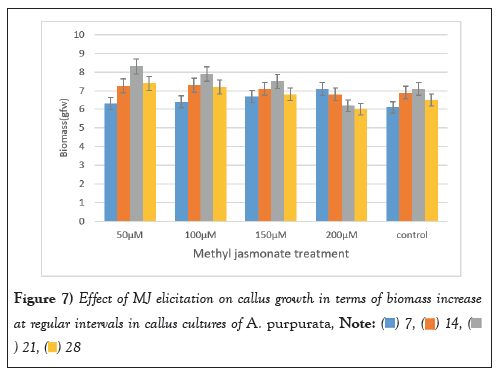
Figure 7: Effect of MJ elicitation on callus growth in terms of biomass increase
at regular intervals in callus cultures of A. purpurata.  .
.
Elicitation
Elicitation experiments were conducted utilizing callus cultures of A. purpurata to investigate the effects of two distinct signaling molecules, MJ and SA. The present study aimed to assess the impact of varying concentrations (50 µM, 100 µM,150 µM and 200 µM) of MJ and SA on the callus culture of A. purpurata over a period of four weeks on biomass and flavonoid production. The control were callus cultures that were not subjected to any elicitor treatment. Following the process of elicitation, the biomass was measured and documented in terms of its Gram Fresh Weight (GFW) on a weekly basis for a duration of four weeks.
After a period of three weeks, a significant augmentation in biomass was seen across different doses of MJ. It is noteworthy that an optimal concentration of MJ (100-150 µM) demonstrated a marginal improvement in growth. The highest increase in biomass, measuring 8.31 ± 1.12 g FW, was seen after a 3-week treatment with a concentration of 50 µM MJ. The most notable increase in biomass was found following a three-week period of stimulation with a concentration of 50 µM. Interestingly, it was shown that greater concentrations of MJ at 200 µM had a detrimental effect on the increase in biomass after a period of four weeks, as depicted in (Figure 7).
The present study investigated the impact of varying doses of MJ on callus growth through the quantification of relative fresh weight and the rate of callus development. The fresh weight of calluses exhibited a reduction in response to the stressor effects of the elicitor, particularly at higher doses. The observed behaviours can be attributed to the inhibitory effects of elicitors on cell proliferation and osmotic adjustment. According to Golkar et al., [49], the heightened demand for turgor in the growth of cells necessitates energy consumption and diminishes the formation of callus. According to the study conducted by Matter, et al. [50], it was shown that higher concentrations of MJ resulted in a decrease in the fresh weight of callus. The highest growth rate (98.21 mg/day) was seen in Dianthus caryophyllus L. callus culture treated with a concentration of 20 M MJ. Abdollahipoor, et al. [51] observed alterations in cell proliferation and callus diameter of Hypericum perforatum following the application of MJ elicitation. In a study conducted by Abdollahipoor et al., [51], it was shown that the callus breadth and fresh weight of Hypericum perforatum calli decreased as the concentrations of MJ increased. The observed differences in variances could perhaps be attributed to variations in the concentration of the elicitor and the specific plant species used in the study [52,53].
The elicitor treated callus was also evaluated for the production of three therapeutically important flavonoids viz Myricetin, Quercetin, and Kaempferol MJ mediated elicitation when applied at different concentrations (50 µM-200 µM) for a duration of 3 weeks evoked a positive response for enhancing the production of all the three flavonoids. Substantial augmentation occurred after 3 weeks with the 100 µM MJ concentration, with myricetin, Quercetin, and Kaempferol content reaching 489 ± 5.99 µg/g DW, 994.22 ± 2.15 µg/g DW, 120.12 ± 2.12 µg/g DW marking a remarkable three-fold increase compared to unstimulated callus cultures. These values were significantly higher than those of the untreated callus cultures, which exhibited a diminishing trend in flavonoid production over time (Figures 8-10).
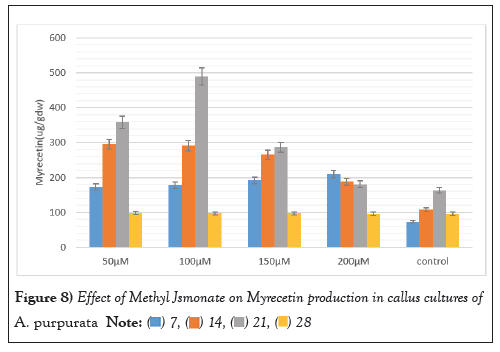
Figure 8: Effect of Methyl Jsmonate on Myrecetin production in callus cultures of A. purpurata.  .
.
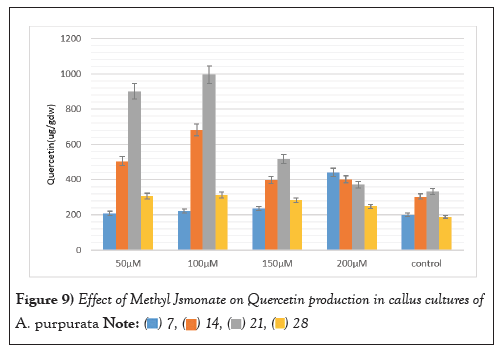
Figure 9: Effect of Methyl Jsmonate on Quercetin production in callus cultures of A. purpurata.  .
.
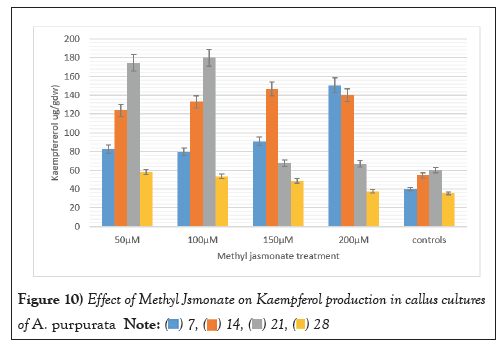
Figure 10: Effect of Methyl Jsmonate on Kaempferol production in callus cultures
of A. purpurata.  .
.
Higher MJ concentrations facilitated a comparatively modest rise in flavonoid production in the fourth week of elicitation. This trend was consistently observed across all three types of flavonoids. These findings underscore the effectiveness of MJ as an elicitor for fostering flavonoid production in A. purpurata.
Salicylic Acid was used as an elicitor at (50 µM-200 µM) for callus cultures of A. purpurata for enhancing the biomass (Figure 11). Remarkably, the most substantial biomass surge was observed after 21 days of stimulation with 50 µM of SA even a modest concentration of SA (150 µM) exhibited a slight growth enhancement, surprisingly, higher SA concentrations exhibited a negative influence on biomass increase. A maximum biomass increase was observed to be 8 ± 1.25 gFW with 50 µM concentration SA after 21 days’ post elicitation treatment.
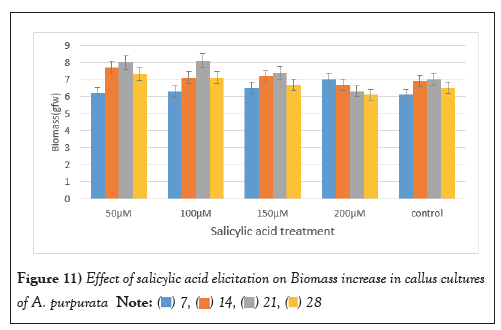
Figure 11: Effect of salicylic acid elicitation on Biomass increase in callus cultures
of A. purpurata.  .
.
Our findings are in corroboration with the reports of Maqbool et al., [54] where he reported the application of SA in Aerva sanguinolenta callus culture resulted in a notable enhancement in the growth rate of callus, as indicated by the higher values of Cell Fresh Weight (CFW), Cell Dry Weight (CDW), and Cell Membrane Stability (CMC), in comparison to the control treatment conditions [54]. The concentration of 0.75 mg/l SA exhibited the most substantial increase in callus growth rate, diameter, and relative fresh weight in Stevia glycoside [55].
SA applied at different concentrations for enhancing the production of flavonoids was analysed at an interval of 7,14, 21 and 28 days. SA at a concentration of 50 µM 21days post elicitation displayed maximum up surge in Myricetin production was 244.62 ± 3.18 µg/g DW. Similarly, the same concentration after 21 days’ post elicitation which also evoked maximum response for production of Quercetin, which was 446.813 ± 1.31 µg/g DW. Maximum Kaempferol production was found to be 60.80 ± 1.21 µg/g DW which is 1.5 folds higher in comparison to unelicited callus cultures (Figures 12-14).
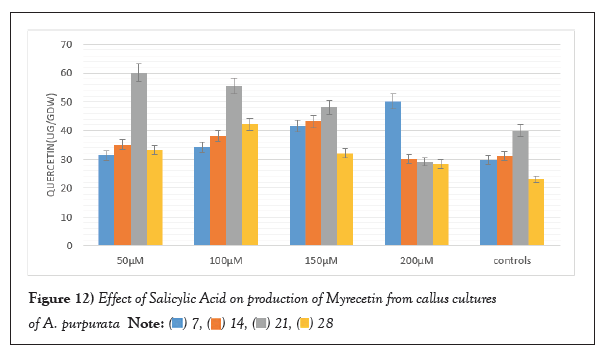
Figure 12: Effect of Salicylic Acid on production of Myrecetin from callus cultures
of A. purpurata.  .
.
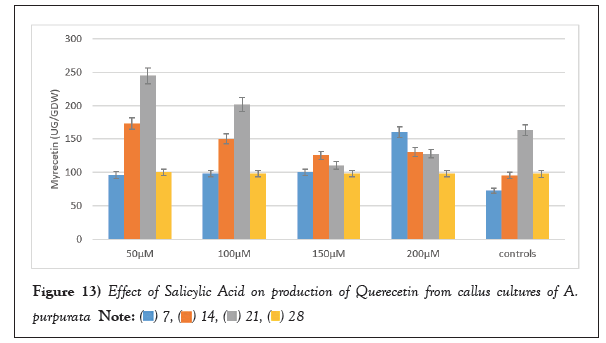
Figure 13: Effect of Salicylic Acid on production of Querecetin from callus cultures of A.
purpurata.  .
.
Figure 14: Effect of Salicylic Acid on production of Kaempferol from callus cultures of A.
purpurata. 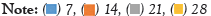 .
.
In callus cultures of Cereus hildmannianus (K.) Schum, elicitation with SA led to a substantial rise the flavonoids with the highest total concentrations were found at 200M SA [56]. The study conducted by Shah, et al. [57] revealed a significant increase in anthraquinone production in Cassia tora callus culture when treated with 10 mM SA. The production of anthraquinone was found to be twice as high compared to the control group.
MJ and SA are exogenous phytohormones which act as a switch for enhancing the yield of secondary metabolites. According to Jayasri, et al. [58], MJ is an effective signaling molecule which initiates the signal transduction pathway for hyperproduction of various secondary metabolites. It is a known fact that SA protects the plant from pathogen attack by its Systemic Acquired Resistance (SAR) mechanism. However, the report given by Hayat, et al. [59] suggested not only its mechanism but also established its role in eliciting the production of secondary metabolites. A recent study on MJ elicitation was given by Hao, et al. [60] on Orostachys cartilaginous A. Bor., in which, it is concluded that elicitation with MJ resulted in highest flavonoid (Kaempferide, Kaempferol-3-o-rutinoside, etc.) production when compared to control. Rezaei, et al. [61] explored the effect of SA and ultrasound on production of taxol results established that SA was more effective than ultrasound in taxol and cell-associated production, this study also suggested the synergism between SA and ultrasound for enhancing the taxol production.
According to Rahmati, et al. [62], the callus culture of Carum carvi L exhibited a significant increase in carvone accumulation, with a 5.55-fold rise (1.83 g g1 DW) and a 2.7-fold increase in limonene (0.62 g g1 DW) at a concentration of 50 M MJ. The impact of MJ on rutin biosynthesis in callus cultures according to a study conducted reported the application of MJ at a concentration of 0.03 mM on 30-day-old callus cultures of Abutilon hirtum L. led to the highest observed rutin content [63]. The study conducted by Restiani et al., [64] revealed that the Talinum paniculatum (Jacq.) callus culture exhibited the highest concentration of flavonoids when treated with 150 M MJ for a duration of 96 hours. Another study given by Chodisetti et al., [65], reported the enhancement of gymnemic acid by MJ and SA elicitation. In which, the highest gymnemic acid content was observed in MJ elicitation when compared to SA. Results of Rezaei et al., [61], revealed that elicitation with SA showed an increase in phenolic content of the cells but a significant reduction in flavonoid content was also observed. Similarly, the present study also revealed the same pattern, where MJ has shown the higher response to flavonoid production (Myrecetin, Quercetin, Kaempferol) when compared to Salicylic acid.
The development of new medications and other products will benefit greatly from the promise shown by callus cultures in the production of novel bioactive chemicals with pharmacological significance. In order to increase the yield of therapeutically useful secondary metabolites from callus cultures, elicitation procedures have proven to be highly effective. Biotechnological strategies that make use of biotic and abiotic elicitors to boost production of bioactive substances have been identified. In this study, MJ and SA are used for the first time as elicitors in A. purpurata callus cultures for enhancing the production of therapeutically important flavonoids. This method was developed to help supply the ever-growing pharmaceutical industry. Elicitation strategy for increasing phyto pharmaceutical production from A. purpurata callus cultures is a new and exciting area of study. Potential strategies for the mass production of high-quality pharmaceutically active flavonoids like Myricetin, Quercetin, and Kaempferol may be suggested by the central role of elicitors and their influence on increasing the harvest index of SMs in A. purpurata. Metabolic engineering of flavonoid production can benefit greatly from transcriptome analysis followed by differential gene expression analysis with elicitated and unelicited callus cultures of A. purpurata.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
Citation: Mounika K, Giri A. Elicitation an approach to improving the metabolic profile of therapeutically important flavonoids from callus cultures of Alpinia purpurata. AGBIR.2024;40(1):792-800.
Received: 23-Nov-2023, Manuscript No. AGBIR-23-120873; , Pre QC No. AGBIR-23-120873 (PQ); Editor assigned: 27-Nov-2023, Pre QC No. AGBIR-23-120873 (PQ); Reviewed: 14-Dec-2023, QC No. AGBIR-23-120873; Revised: 25-Dec-2023, Manuscript No. AGBIR-23-120873 (R); Published: 01-Jan-2024, DOI: 10.35248/0970-1907.24.40.792-800
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
