Agricultural and Biological Research
RNI # 24/103/2012-R1
Research - (2021) Volume 37, Issue 5
Heavy metal pollution is a serious threat to food safety and public Health in the world. Because of this, research was conducted to evaluate the holistic approaches to determine accumulation of heavy metals (Cd, Cu, and Zn) in the soil and their translocation to the wheat crop grown in pots. The soil and wheat crop samples were prepared for heavy metal analysis using acid digestion and heavy metal concentrations were determined using ICP-MS and AAS methods. The results showed that the accumulation of heavy metals in the polluted soil and parts of the wheat plant follows Zn>Cd>Cu. It was observed that Concentrations of Cd and Zn in grain were corresponded with the permissible limits of Cd and Zn in edible plants set by the FAO/WHO: Cd=1.95 and Zn=3.07. The concentration of heavy metals in the samples of wheat plants was also found to follow soil>root>shoot>grain. DGT measure the highest concentration of Cd (16.476 μg/l), Zn (50.0854 μg/l) and Cu (41.2257 μg/l) concentrations in the soil. In wheat plant, the grain is the edible portion that is consumed directly by human. Therefore, the level of heavy metals in grain endangers human health. For that reason, the transferred of metal concentrations in wheat grains should be investigated along with metal concentrations in the roots, shoots and soil, to determine the transport relationship of different HMs within the whole Wheat plant.
Heavy metals (HMs) such as cadmium (Cd), copper (Cu), lead (Pb) and zinc (Zn) are major contaminants which are incorporated into the environments through human activities like: the use of Fertilizer in Agriculture, creation of landfills, urban industrial activities, miming, smelting and sewage sludge applications [1,2]. Due to the toxic nature of these metal elements, its long time persistence in soil can have the ability to be transported into the food chain [3,4]. Persistent HMs pollution in soil do not only degrades the quality of the atmosphere, water bodies, and soil, but also a serious threat to food safety and public health by transport through the food chain [5,6]. As the major sink for HMs in terrestrial ecosystem, soils polluted with HMs have been attracting more and more interests worldwide. However, the assessment of eco-environmental and human risks remains limited. Thus, evaluating the content of their existence in soil and predicting their transfer rates to the food chain is an importance endeavors.
The soil quality guidelines are generally based on the total metal content present within the soil, even though it is generally accepted that, the total metal content in the soil include the available metals and not-available metals fractions, which are closely associated with human health and welfare[7]. To predict these metals fractions, there are several extraction procedures that have been proposed and used to assess metals concentrations in soil and assess their transferred rate from the soil to the food chain via plants uptake [8]. Extraction such as: Aqua regia (HNO3+HCl), HNO3, EDTA, DTPA, CaCl2, HAc and soil solution are soil extraction analysis which have been proposed and most often used in purpose to assess metals available in soil and plants [9]. These procedures have found their application in soil fertility evaluation and for risk assessment of soil contaminant that enters the food chain through plant uptake thereby threatening human and animal’s health [10-12]. DTPA for example, extraction of metal in soil is often used to assess trace elements availability, as this chelator only extract the more “labile” metal forms [13-19].
For that reason, it is understood that within Europe, (for examples, Austria, Denmark, France, Finland, Hungary, Ireland, Norway and Portugal), EDTA extractions of varying concentrations and pH values, and with different additives, are widely used for predicting plant available Cu and Zn in agricultural soil. Even though other extractants are used elsewhere for the same purpose, but EDTA and DTPA are the most commonly used techniques for predicting the availability of micronutrients to plants [20].
However, the lack of agreement in relation to the selection of a universally accepted extractant is perhaps due to the fact that all the extractants propose to assess metals concentration in soils suffer from several weaknesses, which may lead to poor accuracy and robustness. Many extractants are available but there is no agreement on which extractants to use for a particular element. Some countries have more than one soil test depending on the soil type, soil use or geographical area.
Other measures such as the concentration in soil solution or in weak salt extraction have also been proposed as indicators of plant availability, since plant access mineral elements from the soil solution [21-28]. Reviewed literature covering a range of extractants and plant species and found that no relationship could be established between any extractant and plant shoot Cu. They reported that EDTA and DTPA were poor predictors of the plant available Zn [29,30].
Cadmium is a toxic element in fertilizers where the conventional extraction methods often fail to give an accurate assessment of its availability to plant [31,32]. It is important therefore, to developed new methods that better shows the accurate amount of these available metals in the soil to be able to assess the transferred rate to the food chain. The diffusive gradient in thin films (DGT), which is a diffusive method that accumulates dissolved metals, sulphides and phosphates in soil, is one new method.
According to Tandy, DGT can in a better way show plant available nutrients compared to the standard extraction methods, which show both plant available and not direct plant available nutrients. More research is needed to predict heavy metal uptake as a function of soil solution concentration. In turn, this can be used to set limits on the level of metals in soil and their transport to the food chain.
Therefore, the objective of the study was to investigate and assess the heavy metals content in different parts of durum Wheat plant. To investigate the accumulations of Cd, Cu, and Zn in wheat plant parts considering the measurements of (DGT, HNO3, CaCl2, acetic acids (HAc) and soil solution); to assess the effect of (DGT, HNO3, CaCl2, acetic acids (HAc) and soil solution) holistically from the soil properties and to investigate the correlation between the concentrations of Cd, Cu, and Zn in the root, Shoot and grain of durum wheat grown in pots, and the concentrations of the Heavy metals in acetic acid (HAc), HNO3, and CaCl2 and concentrations measured by DGT.
Study site and sample preparation
This study was conducted at the Teaching and Practice Base for plants nutrition and fertilizers and micro-elements research center of Huazhong Agricultural University, Hubei Province, Wuhan, China.
The Loessal soil for the study was collected from an agricultural field trail in Shaanxi, china; only the topsoil (0-25 cm) was collected, and soil samples were taken with soil auger. Zn and Cu were selected because of their high mobility in the soil and Cd was selected due to its high toxicity and also because of the existent interrelationship with Zn. There were eight treatments levels of Zn, Cu and Cd.
Experimental materials and setup
The plastic experimental pots, approximately, 10.5 L was lineup and were filled with 4 kg of soils wetted with 500 ml of solution. The pots were left to incubate for two weeks. After that, the pots were fertilized and left for another one week with a soluble minerals fertilizer before planting the wheat. The formula was H2NCONH2, Ca (H2PO4) and KCl. Briefly, 2 g of each fertilizer was weighed into a pot in combinations, making each pot receiving the total of 6 g per pot for all the treatments. The pots were seeded with durum variety of winter wheat (Triticum aestivum L.). The seeds were soaks in distilled water for 4 days before seeding. All the Pots were seeded with 13 seeds and after germination; the plants were thinned to ten plants per pot. A growth chambers with a temperature and humidity control were used. The light intensity was kept at 450 μ Lux. The temperature was kept at 24°C with 12 hours daylight and 11 hours darkness and relative humidity at 80% respectively. The Cu, Zn and Cd combinations were (mg/l): 8 Cd, 300 Cu, and 25 Zn; 8 Cd/25 Zn, 300 Cu/25 Zn, and 8 Cd/300 Cu and 8 Cd/25 Zn/300 g Cu.
Method
The experimental design was a complete factorial with 8 treatments, 4 replicates of each treatment. The treatments consisted of one level of Cu, Zn and Cd and a control with no Cu, Zn and Cd addition. For Cd alone, only one level of 8 mg/kg was tested. For Zn alone, only one level of 25 mg/kg was tested and same for Cu alone. Combinations of Cu, Cd and Zn were: 8/25 mg/kg, 300/25 mg/kg, and 8/300 mg/kg and 8/25/300 mg/kg respectively. All pots were filled with soil and the solution added, numbered and randomly assigned a treatment. During harvest, the plant samples were up-rooted and gathered near the edge of each experimental pot, and the wheat shoots and roots were thoroughly rinsed with distilled water and separated. The roots were further washed in an ultrasonic system with deionized water to remove fine soil particles. After collecting all the 32 soil samples from each pot (8 treatments × 4 replicates) and plant samples, they were all dried for approximately 48 hours at 60ºC. About, 900 grams soils were sieved to <2 mm and stored in polythene bags and kept at room temperature for further analysis. The plant samples were then grinded into small pieces in a grinder (Retsch GM200) using a titanium blade to avoid contamination and about, 60 grams wheat was stored in a polythene plastic and then prepared for further analyses in the lab.
Heavy metal extraction
The procedures HAc, CaCl2, DGT, soil solution and HNO3 were used to extracts Copper (Cu), Zinc (Zn) and Cadmium (Cd) from the soil (Table 1).
| Elements | Weak extraction | Strong extraction | Diffusive method |
|---|---|---|---|
| Cu | CaCl2 | HNO3, HAC | DGT |
| Cd | CaCl2 | HNO3, HAC | DGT |
| Zn | CaCl2 | HNO3, HAC | DGT |
Table 1: The used extraction procedures and measured metals.
Soil chemical analysis
The soil pH was determined by an 8:2 solution ratio using distilled water [33]. The cation-exchange capacity (CEC) was determined using the ammonium acetate (1 M and pH 7.0) method [34]. The organic matter content (OM) was determined by using K2Cr2O7 wet oxidation method. The soil texture was determined by using the pipette method [35]. The elemental analysis was determined by the microwave digestion method that consisted to use aqua regia (Guaranteed reagent) and hydrofluoric acid (Guaranteed reagent) for the digestion of 20 mg of samples. The concentration of Al, Ca, Cr, fe, K, Li, Mg, Mn, Na, Ni, and Pb were determined using inductively coupled plasmaoptical emission spectroscopy (ICP-OES Visata-MPX, Varian, California, U.S.A) [36-38].
DGT method
The DGT application was done according to “the practical guide for using DGT in soils” provided by DGT research Ltd., Lancaster, and Nanjing where the DGT devices were ordered from. A mixed binding layer (MBL) was used. The DGT samples were run in two phases because of doubts whether the method would work or not. Phase one was done with two replicates from each treated soil and eluted in HCl. Phase two was done with the two remaining replicates from each treated soil and eluted in HNO3. About 50.0 g of dry soil was weighted and placed in 250 ml containers and then distilled water was carefully added until the soil reached the saturation point, about 70% ml per container. The containers were left to calibrate for 24 h in room temperature (24-25°C). In DGT phase1 the devices were lids slightly open to avoid anaerobic conditions and in DGT phase 2 the devices were press gently into the soil using twist and turns methods. The DGT devices were stored in the fridge, before deployment, the devices were left to acclimatize to room temperature for about 3 hours before deployment into the soils. The exposure window of the DGT was smeared with moist soil just before it was pushed to the soil in the containers just to ensure good contact with the soil. The exposure window was smeared with a knife. The DGT devices were left in the soil for about 24 h. Then the devices were removed from the soil, adhering soil that remained on the devices were rinsed with distilled water and dried easily with tissues. There after the cap of the DGT devices was open by a sharp knife and removed. During this process, the knife was cleaned with ethanol between every treatment, it is very important to work in a clean environment so the DGT devices cannot get contaminated. The binding gel was then removed by a forceps and eluted in 2 ml 1 M HCl and thereafter in 1 M HNO3. The gels were removed from the elution solution after 24 h and were analyzed by ICP-MS.
Soil solution method
This method was a solid-to-liquid ratios by 1:10 was used; 1 gram of air-dry soil was weighed into a plastic tube. Than 10 ml of distilled water was added and shake into the shaker for 2 hours with 180 rpm at 30°C. Thereafter, the solutions were centrifuged with 2800 rpm for twenty (20) minutes, hence each sample were filter through whatman No. 40 filter paper into 10 ml plastic bottles. All the samples were done at the same time. The samples were analyzed using Atomic Absorption Spectrometry analyzer (AAS).
CaCl2 method
In this method, a solid-to-liquid ratio by 1:10 was used; 1 gram of air-dry soil was weighed and placed into a plastic tube. Then 10 ml of 0.01 M CaCl2 added (8.82 g CaCl2 was dissolved in 6 liters of distilled water) into the tubes and did shake in a shaker for two hours at 30°C with 180 rpm. Thereafter centrifuged with 2800 rpm about twenty (20) minutes, hence each sample was filtered through Whatman No. 40 filter paper into 10 ml plastic bottles. All the samples were done at the same time plus two CaCl2 blanks. The samples were then sent to be analyzed by AAS analysis.
Acidic acid extraction method
In this method, a solid-to-liquid ratio by 1:10 was used; 1 gram of air-dry soil was weighed into a plastic tube. Than 10 ml of 0.11 M (HAC) was added into the tubes and shake it into the shaker for 2 hours at 30° C with 180 rpm. Thereafter, the solutions was centrifuged with 2800 rpm for twenty (20) minutes, hence each sample was filtered through what man No. 40 filter paper into 10 ml plastic bottles. All the samples were done at the same time. The samples were later sent to be analyzed by AAS analysis.
HNO3 method
Two grams of soil was weighed into a 50 ml conical flask, hence added 10 ml 65% HNO3, the samples were left to washed down and placed on the heat bed in the digestion room for 72 hours at 220°C. Thereafter, the samples were allowed to cool and shaken briefly by hand, 5 ml 65% HNO3 was added and heated for another 8 hours. Afterward, the samples were allowed to cool down and diluted with 25 ml distilled water; the samples were filter through what man No. 40 filter paper into 10 ml plastic bottles. All the samples were done at the same time. After that, the samples were analyzed with AAS analyzer.
Plant analysis
One gram of wheat (roots, shoots and grains) was weighed into a 50 ml conical flask, hence added 10 ml 65% HNO3, thereafter the samples were placed on the heat plate in the digestion room and was heated in three steps: 100 degrees for 24 hours, 120 degrees for 16 hours and 130 degrees for 10 hours. Thereafter, the samples were allowed to cool down, briefly shaken by hand and then further added with 5 ml 65% HCl and heated for another 10 hours with 130 degrees, the samples was allowed to cooled down and diluted with 25 ml of distilled water. The samples were then filtered through what man No. 40 filter paper into 10 ml plastic bottles. All the samples were done at the same time. Afterward, the samples were analyzed with AAS analyzer.
Statistical analysis
All the collected data was firstly written and calculated in Excel. The statistical analyses were performed using SPSS Version 22 for Windows. The significance of differences between the means of treatments (four replicates) was evaluated using ANOVA, followed by Duncan’s multiple range tests at (p<0.05). Correlations between extractable heavy metal concentrations in soils and the content of heavy metals in roots, shoots and grains of wheat plant were evaluated using Pearson correlation analysis. Regression analyses was carried out to establish the relationships between parameters measured for predictive purposes.
The study soil contained moderate amount of clay (32.8%) with moderate amount of organic matter ranging from (27.89% to 28.58%). The pH value ranges from (6.1 to 7.4) in the soil. The experiment was factorial (randomize complete block design) consisting of eight treatments with four (4) replicates of each treatment. There was a wide difference between the concentrations of Cu, Zn and Cd extracted between different extractions procedures and the treatments contained (Tables 2-4). There was also a wide variation between the different treatments and the mean value was calculated of the four replicates from each treatment in the field. The grown wheat species was Durum wheat (Triticum durum L.) winter wheat.
| Treats | Soil solution | CaCl2 | HAc | HNO3 | DGT µg/l | Cd uptake (mg/kg) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Roots | Shoots | Grains | ||||||||
| T1 | 0.0024b | 0.0293c | 0.0015d | 0.0406d | 1.9501b | 0.0067d | 0.0447c | 0.0486c | ||
| T2 | 0.0109a | 0.7418a | 0.2269a | 0.7336b | 14.5881a | 0.1599a | 0.0453a | 1.9780a | ||
| T3 | 0.0027b | 0.0121b | 0.0027d | 0.0488d | 2.0661b | 0.0048d | 0.0541b | 0.0156d | ||
| T4 | 0.0033b | 0.0569b | 0.0023d | 0.0309e | 2.0512b | 0.0037d | 0.0076c | 0.0153d | ||
| T5 | 0.0031b | 0.0018c | 0.1460b | 0.3938c | 16.4761a | 0.1095b | 0.0153e | 1.9780a | ||
| T6 | 0.0012c | 0.0012c | 0.1263c | 0.2983c | 14.5503a | 0.1507a | 0.0254d | 1.9630a | ||
| T7 | 0.0023d | 0.0018c | 0.0022d | 0.0327e | 0.6134 b | 0.0039d | 0.0051f | 0.0921b | ||
| T8 | 0.0050b | 0.0014c | 0.1324b | 2.0407a | 16.2848a | 0.0680c | 0.0230d | 1.9667a | ||
Note: Values of the same column with the same exponential letter are not statistically different at (P<0.05).
Table 2: Cd uptake by Durum Wheat plant (mg/kg) and concentration in soil extracted by DGT, HAc, CaCl2, HNO3 and Soil solution
| Treats. | Soil solution | CaCl2 | HAc | HNO3 | DGT µg/l | Zn uptake (mg/kg) | ||
|---|---|---|---|---|---|---|---|---|
| Roots | Shoots | Grains | ||||||
| T1 | 0.0294d | 0.0294a | 0.0260c | 0.0526c | 41.0079a | 0.0617d | 0.0312d | 0.0284c |
| T2 | 0.0345c | 0.0774a | 0.0231c | 0.0796c | 36.0836b | 0.0436d | 0.0377d | 0.0412c |
| T3 | 0.0353c | 0.0341a | 0.0308c | 0.0257c | 48.1836a | 0.0471d | 0.0358d | 0.0526c |
| T4 | 0.0547a | 0.0365a | 0.0993a | 3.5390a | 50.0854a | 0.8948a | 0.3203a | 3.0721a |
| T5 | 0.0350c | 0.0267a | 0.0251c | 0.0364c | 22.0613c | 0.0420d | 0.0233d | 0.0585c |
| T6 | 0.0314c | 0.0408a | 0.0203d | 2.7812b | 29.1014c | 0.4191c | 0.2076b | 2.5681b |
| T7 | 0.0473a | 0.0683a | 0.0715b | 3.0304b | 40.9708a | 0.6400b | 0.0870c | 2.9802a |
| T8 | 0.0390b | 0.0608a | 0.0185d | 2.7714b | 34.4697b | 0.4311c | 0.1462b | 2.6148b |
Note: Values of the same column with the same exponential letter are not statistically different at (P<0.05).
Table 3: Zn uptake by Durum Wheat plant (mg/kg) and Cu concentration in soil extracted by DGT, CaCl2, HNO3 and Soil solution.
| Treats | Soil solution | CaCl2 | HAc | HNO3 | DGT µg/l | Cu uptake (mg/kg) | ||
|---|---|---|---|---|---|---|---|---|
| Roots | Shoots | Grains | ||||||
| T1 | 0.0219b | 0.0101a | 0.0086b | 0.0219c | 6.6908c | 0.1473d | 0.0273a | 0.0986b |
| T2 | 0.0250b | 0.0051c | 0.0060d | 0.0291c | 9.3112b | 0.0094d | 0.0303a | 0.0213c |
| T3 | 0.0056a | 0.0021d | 0.0194a | 0.1703a | 41.2257a | 1.2325a | 0.0298a | 0.2711a |
| T4 | 0.0278b | 0.0037c | 0.0065c | 0.0470c | 9.3302b | 0.1738d | 0.0298a | 0.0404b |
| T5 | 0.0309a | 0.0079b | 0.0106b | 0.1256b | 34.5397a | 0.4384c | 0.0260a | 0.2342a |
| T6 | 0.0263b | 0.0042c | 0.0084b | 0.0266c | 16.7288b | 0.1155d | 0.0308a | 0.0240b |
| T7 | 0.0457a | 0.0021d | 0.0123b | 0.1384b | 38.1744a | 0.8181b | 0.0351a | 0.2307a |
| T8 | 0.0408a | 0.0017e | 0.0125b | 0.1089b | 35.4997a | 0.4385c | 0.0359a | 0.2199a |
Note: Values of the same column with the same exponential letter are not statistically different at (P<0.05).
Table 4: Cu uptake by Durum Wheat plant (mg/kg) and Cu concentration in soil extracted by DGT, CaCl2, HNO3 and Soil solution.
Cupper (Cu) uptake
The extracted amount of Cu differed between the treatments and the different methods of extraction of analysis. The highest concentration of plant Cu was obtained at Treatment-3 Cu alone 1.235 mg/kg closely followed by Treatment-7 Cu/Zn combined and Treatment-5 Cu/Cd combined with values ranging from 0.098 to 0.0871 mg/kg respectively for roots. The highest was recorded for grains at Treatment-3 Cu alone 3.072 mg/kg. The least Cu concentration was measure in Treatment-1 0.047 mg/kg followed by Treatment-2 Cd alone and Treatment-4 Zn alone 0.023 & 0.044 mg/kg. DGT measure the highest amount of Cu in soil at Treatment-3 Cu alone 41.257 μg/l closely followed by Treatment-7 Cu/Zn combined, Treatment-8 Cu/Cd/Zn combined and Treatment-5 Cu/Cd, with values ranging from 38.174, 35.497 and 34.597 μg/l respectively. CaCl2, HAc and HNO3 showed the highest extracted concentration of Cu at Treatment-5 Cu/Cd 0.079 mg/ kg (CaCl2), Treatment-6 Cd/Zn 0.084 mg/kg (HAc) and Treatment-3 Cu alone 0.173 mg/kg (HNO3). During the plant growth period, no plant shows the symptoms of Cu stress, which indicates that the concentration of Cu was above the deficient threshold of 1.3 mg/kg in the soil.
Correlation between plant uptake and soil Extractable Cu
HNO3, HAc, CaCl2 and DGT measurements had a significant correlation with Cu uptake by the plant at (p<0.001). The values for the R2 differed however between treatments and the extracted procedures were. CaCl2 (R2=0.461) showed a very poor correlations than DGT, HNO3, HAc and soil solution at (p<0.001 (R2=0.823***, 0.751***, 0.776*** (Figure 1).
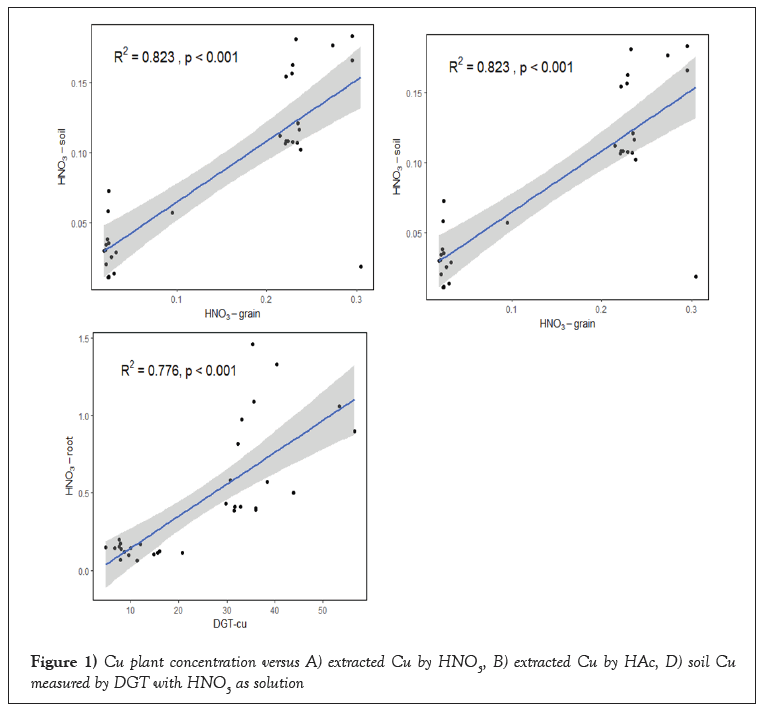
Figure 1: Cu plant concentration versus A) extracted Cu by HNO3, B) extracted Cu by HAc, D) soil Cu measured by DGT with HNO3 as solution
Correlation between measured Cu by DGT vs. HAc, CaCl2, HNO3 and Soil solution
The Cu measured by the DGT technique showed a very strong and significant correlation with both HAc extractable Cu. (p<0.001; R2=0.875); and Soil Solution extractable Cu (p<0.001; R2=0.856). Significant correlation was observed for HNO3 extractable Cu and DGT Cu measure (R2=0.875; p<0.001). CaCl2 was significant at (R2=-0.466, p<0.01) (Figure 2).
Figure 2: Relationship between extractable Cu by DGT (μg/l) versus extractable Cu by A) Soil Solution, CaCl2 and B) HNO3 (mg/kg). The blue line in the figure shows the standard deviation.
Zinc (Zn) uptake
The amount of Zn extracted differed between treatments and the different extraction procedures for soil analysis. The highest plant Zn concentration in the root was obtained at T4 Zn alone 0.898 mg/kg closely followed by T7 Cu/Zn 0.640 mg/kg, while the least amount of Zn. was extracted from T6 Cd/Zn 0.419 mg/kg followed by T8 Cd/Zn/Cu 0.431 mg/kg; for shoot, the highest Zn was recorded at T4 Zn alone 0.323 mg/kg closely followed by T6 Cd/Zn 0.276 mg/kg, while the least was recorded at T8 Cu/Cd/Zn 0.146 mg/kg. For grains, the highest plant Zn concentration was at T4 Zn alone 3.072 mg/kg closely followed by T7 Cu/Zn 2.982 mg/kg, T8 Cu/Cd/ Zn 2.648 mg/kg and T6 Cd/Zn 2.568 mg/kg respectively. For the metals applied in combination, the level of Cd lead to lower Zn plant uptake, for this experiment at T6 Cd8/25 Zn. Zn uptake was more noticeable under higher plant growth to where Zn was applied alone as treatment. Zn plant uptake reached the peaked for Zn solution levels of 25 mg/l. The Zn alone as treatment registered the highest uptake of Zn at 3.0721 mg/Kg of plant uptake at T4. Plant Zn and Cd uptake was not significantly different at T6 Cd8/25 Zn mg/kg, because plant uptake had probably reached its limit. The lowest uptake of Zn was obtained by T6 Cd8/25 Zn 0.419 mg/kg followed by T8 Cd8/25 Zn/300 Cu 0.431 mg/kg in root; for shoot the highest Zn was recorded at T4 25 Zn alone as treatment 0.323 mg/kg closely followed by T6 Cd8/25 Zn 0.276 mg/kg, while the least was recorded at T8 Cu300/ Cd8/25 Zn 0.146 mg/kg. For grains, the highest plant Zn concentration was at T4 Zn alone as treatment 3.072 mg/kg closely followed by T7 Cu 300/25 Zn 2.982 mg/kg, T8 Cu300/Cd 8/25 Zn 2.618 mg/kg and T6 Cd 8/25 Zn 2.568 mg/kg respectively. Treatment (1) showed significantly the lowest uptake of Zn, Cd and Cu at 0.032, 0.067, 0.027 mg/Kg, of plant uptake. The Diffusive gradients in thin films technique extracted the highest amount of Zn 50.0854 μg/l at T4 Zn alone closely followed by T3 Cu alone 48.1836 μg/kg, T1 41.079 μg/kg and T7 Cu/Zn 40.978 μg/kg, while the least was recorded at T5 Cu/Cd 22.0613 μg/kg; Whereas HAc extracted the most Zn 0.099 mg/kg at T4 Zn alone and the least 0.085 mg/kg at T8 Cu/ Cd/Zn. The highest concentration of Zn extracted by HNO3 was in T4 Zn alone 3.539 mg/kg closely followed by T7 Cu/Zn 3.034 mg/kg and the least amount of Zn was extracted in T6 Cd/Zn 2.782 mg/kg and T8 Cu/Cd/Zn 2.774 mg/kg respectively. During the plant growth period, none of the plant shows Zn stress, which indicates that the concentration of Zn was above the deficient threshold of 14 mg/kg in the soil.
Correlations between plant uptake and extractable Zn
There was no significant correlation between DGT and HNO3 measurements of extractable Zn (p>0.001). HAc on the other hand showed a significant correlation with Zn plant uptake (p<0.001; R2=0.788). Significant correlation existed between HNO3 extractable Zn and HNO3 Zn plant uptake (p<0.00, R2=0.990). The blue lines in the graphs below indicate the standard deviation (Figure 3).
Figure 3: Relationship between extractable Zn by HNO3 versus extractable Zn by A) HAc and B) HNO3 (mg/kg)
Correlations between measured Zn by DGT vs. HAc, CaCl2 and HNO3 and Soil Solution
Diffusive gradients in thin films technique extractable Zn did not show a significant correlation with CaCl2 or HNO3 extracted Zn (p=0.355; p=0.9053). Significant correlation was found between DGT-Zn and Soil Solution Zn (R2=0.515, p <0.01). DGT-Zn and HAc also show significant.
Cadmium (Cd) uptake
The amount of extracted Cd differed both between treatments and the different extraction methods for soil analysis. The highest plant Cd concentration was obtained at T2 Cd (1.978 mg/kg) and Treatment-5 Cu/ Cd 1.978 mg/kg closely followed by Treatment-6 Cd/Zn 1.963 mg/kg and T8 8 Cd/Cu300/25 Zn 1.967 mg/kg in the grain respectively, while the lowest concentration of Cd was obtained at Treatment-2 Cd 0.159 mg/kg followed by T6 Cd8/25 Zn 0.157 mg/kg in the roots. CaCl2, HAc, HNO3 and DGT measurements of Cd had highest concentration of Cd in T2 Cd (CaCl2=0.748 mg/kg, HAc=0.269 mg/kg, HNO3=2.047 mg/kg and DGT=16.284 μg/l. Both HNO3=0.736 mg/kg and DGT 1.951 μg/l showed the lowest concentration of Cd in Treatment-8 Cd/Cu/Zn.
Correlation between plant uptake and extractable Cd
There was a significant relationship between all of the extraction methods use for the soil analysis (HNO3, HAc, and DGT) and plant uptake of Cd at (p<0.001) (Figures 4). On the other hand, CaCl2 show strong correlation at (P<0.001). On the other hand DGT show poor correlation with HNO3 for plant shoot Cd uptake (R2=-0.137, p=0.4531).
Figure 4: Zn plant concentration versus A) extracted Zn by DGT, B) extracted Zn by HAc, C) soil Zn measured by DGT with HNO3 as elution solution
Zinc (Zn) uptake
The amount of Zn extracted differed between treatments and the different extraction procedures for soil analysis. The highest plant Zn concentration in the root was obtained at T4 Zn alone 0.898 mg/kg closely followed by T7 Cu/Zn 0.640 mg/kg, while the least amount of Zn. was extracted from T6 Cd/Zn 0.419 mg/kg followed by T8 Cd/Zn/Cu 0.431 mg/kg; for shoot, the highest Zn was recorded at T4 Zn alone 0.323 mg/kg closely followed by T6 Cd/Zn 0.276 mg/kg, while the least was recorded at T8 Cu/Cd/Zn 0.146 mg/kg. For grains, the highest plant Zn concentration was at T4 Zn alone 3.072 mg/kg closely followed by T7 Cu/Zn 2.982 mg/kg, T8 Cu/Cd/ Zn 2.648 mg/kg and T6 Cd/Zn 2.568 mg/kg respectively. For the metals applied in combination, the level of Cd lead to lower Zn plant uptake, for this experiment at T6 Cd8/25 Zn. Zn uptake was more noticeable under higher plant growth to where Zn was applied alone as treatment. Zn plant uptake reached the peaked for Zn solution levels of 25 mg/l. The Zn alone as treatment registered the highest uptake of Zn at 3.0721 mg/Kg of plant uptake at T4. Plant Zn and Cd uptake was not significantly different at T6 Cd8/25 Zn mg/kg, because plant uptake had probably reached its limit. The lowest uptake of Zn was obtained by T6 Cd8/25 Zn 0.419 mg/kg followed by T8 Cd8/25 Zn/300 Cu 0.431 mg/kg in root; for shoot the highest Zn was recorded at T4 25 Zn alone as treatment 0.323 mg/kg closely followed by T6 Cd8/25 Zn 0.276 mg/kg, while the least was recorded at T8 Cu300/ Cd8/25 Zn 0.146 mg/kg. For grains, the highest plant Zn concentration was at T4 Zn alone as treatment 3.072 mg/kg closely followed by T7 Cu 300/25 Zn 2.982 mg/kg, T8 Cu300/Cd 8/25 Zn 2.618 mg/kg and T6 Cd 8/25 Zn 2.568 mg/kg respectively. Treatment (1) showed significantly the lowest uptake of Zn, Cd and Cu at 0.032, 0.067, 0.027 mg/Kg, of plant uptake. The Diffusive gradients in thin films technique extracted the highest amount of Zn 50.0854 μg/l at T4 Zn alone closely followed by T3 Cu alone 48.1836 μg/kg, T1 41.079 μg/kg and T7 Cu/Zn 40.978 μg/kg, while the least was recorded at T5 Cu/Cd 22.0613 μg/kg; Whereas HAc extracted the most Zn 0.099 mg/kg at T4 Zn alone and the least 0.085 mg/kg at T8 Cu/Cd/Zn. The highest concentration of Zn extracted by HNO3 was in T4 Zn alone 3.539 mg/kg closely followed by T7 Cu/Zn 3.034 mg/kg and the least amount of Zn was extracted in T6 Cd/Zn 2.782 mg/kg and T8 Cu/Cd/Zn 2.774 mg/kg respectively. During the plant growth period, none of the plant shows Zn stress, which indicates that the concentration of Zn was above the deficient threshold of 14 mg/kg in the soil.
Correlations between plant uptake and extractable Zn
There was no significant correlation between DGT and HNO3 measurements of extractable Zn (p>0.001). HAc on the other hand showed a significant correlation with Zn plant uptake (p<0.001; R2=0.788). Significant correlation existed between HNO3 extractable Zn and HNO3 Zn plant uptake (p<0.00, R2=0.990). The blue lines in the graphs below indicate the standard deviation (Figure 3).
Correlations between measured Zn by DGT vs. HAc, CaCl2 and HNO3 and Soil Solution
Diffusive gradients in thin films technique extractable Zn did not show a significant correlation with CaCl2 or HNO3 extracted Zn (p=0.355; p=0.9053). Significant correlation was found between DGT-Zn and Soil Solution Zn (R2=0.515, p <0.01). DGT-Zn and HAc also show significant.
Cadmium (Cd) uptake
The amount of extracted Cd differed both between treatments and the different extraction methods for soil analysis. The highest plant Cd concentration was obtained at T2 Cd (1.978 mg/kg) and Treatment-5 Cu/ Cd 1.978 mg/kg closely followed by Treatment-6 Cd/Zn 1.963 mg/kg and T8 8 Cd/Cu300/25 Zn 1.967 mg/kg in the grain respectively, while the lowest concentration of Cd was obtained at Treatment-2 Cd 0.159 mg/kg followed by T6 Cd8/25 Zn 0.157 mg/kg in the roots. CaCl2, HAc, HNO3 and DGT measurements of Cd had highest concentration of Cd in T2 Cd (CaCl2=0.748 mg/kg, HAc=0.269 mg/kg, HNO3=2.047 mg/kg and DGT=16.284 μg/l. Both HNO3=0.736 mg/kg and DGT 1.951 μg/l showed the lowest concentration of Cd in Treatment-8 Cd/Cu/Zn.
Correlation between plant uptake and extractable Cd
There was a significant relationship between all of the extraction methods use for the soil analysis (HNO3, HAc, and DGT) and plant uptake of Cd at (p<0.001) (Figures 4). On the other hand, CaCl2 show strong correlation at (P<0.001). On the other hand DGT show poor correlation with HNO3 for plant shoot Cd uptake (R2=-0.137, p=0.4531).
Correlations between measured Cd by DGT vs. HAc, CaCl2, HNO3 and Soil Solution
Significant correlations were established for Cd in all the extraction methods at (p<0.001; p<0.01). On the other hand, DGT and CaCl2 show a very poor correlation for measured Cd (Tables 2-4) shows the correspondent plant Zn, Cu and Cd uptake for each treatment level and extraction methods. The combination of Cu/Cd stimulated the uptake of Cd at T5 in this experiment. The highest concentration of plant Cu was obtained at T3 1.235 mg/kg closely followed by T7 Cu/Zn and T5 Cu/Cd with values ranging from 0.818-0.438 mg/kg respectively for roots. The highest was recorded for grain at T3-Cu alone (0.2711 mg/kg) closely followed by T5 Cu/Cd 0.234 mg/kg. The least Cu concentration was measure at T1 0.098 mg/kg followed by T2 Cd alone and T4 Zn alone 0.023 & 0.044 mg/kg. The Diffusive gradients in thin films (DGT) measure the highest amount of Cu in the soil at T3 Cu alone 41.257 μg/l closely followed by T7 Cu/Zn, T8 Cu/Cd/Zn and T5 Cu/Cd, with values ranging from 38.174, 35.499 and 34.597 μg/l than the conventional methods. The combination of Cu/Zn/Cd at T8 had adverse effects on the uptake of Cd by roots (Figure 5). In combination, Cu stimulated Cd uptake by plant at T5 Cu/Cd uptake of heavy metals by roots and Shoots). Cd and Zn show the strongest antagonistic effect, indicating that Zn uptake in the shoot is increased by Cu addition and that Zn alone was not toxic but became overtly toxic when present with Cu. standard deviations of four replications of the trail). Bars with the same letters are not significantly different according to Duncan’s multiple range tests at p≤0.05. The above graph shows the Uptake of Cd, Cu and Zn by roots in durum wheat. This distribution of heavy metals in the wheat plant reveals that plant roots are in direct contact with the soil. As a result, the concentration of heavy metal is usually higher in the roots than in the other plant parts. The heavy metal concentration is higher in the root and grain than in the shoot. The HMs applied separate recorded the highest amount than in combination for all the treatment levels in the root. With the combination of metal elements, some interaction was observed as antagonistic and synergetic (Figure 6).
Figure 5: Cd plant concentration versus A) extracted Cd by CaCl2 B) extracted Cd by HNO3 and D) Cd concentration in the soil measured by DGT
Figure 6: Correlation between extractable Cd by DGT (μg/l) vs extractable Cd by A) Soil Solution (mg/kg) and B) HAc (mg/kg) and C) HNO3 (mg/kg)
Most recently, An, Kim et al. reported antagonistic effects between Zn and Cd; the applied Zn reduced the plant uptake of Cd. Among all the HMs, Cd and Zn show the strongest antagonistic effect, indicating that Zn uptake in the shoot is increased by Cu addition and that Zn alone was not toxic but became overtly toxic when present with Cu (Figures 7 and 8).
Figure 7: The uptake of HMs by root from different treatments in wheat plant. Ck: Soil/mineral fertilizer, Cd: 8 mg, Zn: 25 mg, Cu: 300 mg. (Values are expressed as means ± standard deviations of four replications of the trail). Bars with the same letters are not significantly different according to Duncan’s multiple range tests at p≤0.05
Figure 8: The uptake of HMs by shoot from different treatments in wheat plant. Ck: Soil/mineral fertilizer, Cd: 8 mg, Zn: 25 mg, Cu: 300 mg. (Values are expressed as means ± standard deviations of four replications of the trail). Bars with the same letters are not significantly different according to Duncan’s multiple range tests at p≤0.05
The above graph shows the accumulation of Cd, Cu and Zn by shoot in durum wheat plant. Even though the amount of Cd accumulated in the shoot tissues differed between treatments, but the relative pattern of Cd accumulation in the shoot was similar (Table 2). Unlike Cu, the largest pools of Cd and Zn in the shoots were in the grains. Cadmium, in contrast to the micronutrients (Cu, and Zn), was retained mostly in the shoots. The accumulation of Cadmium in the plants tissues is influenced by numerous factors, including available Cd in the soil, soil type and chemistry, climate, agronomic practices, and the plant species. The accumulation of heavy metals by grains with in treatments. In wheat plant, the grain is the edible portion that is consumed directly by human (Figure 9). Therefore, the level of heavy metals in grain endangers human health. For that reason, the transferred of metal concentrations in wheat grains should be investigated along with metal concentrations in the roots and shoots and soil, to determine the transport relationship of different HMs within the whole Wheat plant. In this study, in contrast to the micronutrients such as Cu and Zn, Cadmium was largely taken in the grains. In contrast to Cd, the accumulation of micronutrients occurred mostly in the shoots and subsequently in the grains compared to Zn. Larger concentration of Zn was recorded in the grain to where Zn was applied separately as treatment. The concentration of Cd in the mature grains was similar between treatments, with the highest being recorded to where it was applied separately and in combination with Cu. The combined and single application rate of elements provokes the increase of Cd and Zn concentration in the grain.
Figure 9: The accumulation of HMs from different treatments in the wheat grain. Ck: Soil/mineral fertilizer, Cd: 8 mg, Zn: 25 mg, Cu: 300 mg. (Values are expressed as means±standard deviations of four replications of the trail). Bars with the same letters are not significantly different according to Duncan’s multiple range tests at p≤0.05
Since the transport of Cd, Cu and Zn to the roots and shoots was found to be small, only uptake into the grains was examined further due to the risk these heavy metals transfer to the food chain thereby threatening human and animal health. As regarding to different metals, Cd, a toxic element, is readily taken up by crops and can be transported to edible parts where it can be accumulated to a relatively high levels. Therefore, assessing the uptake and risk associated with these heavy metals accumulation in cultivated food crops such as Wheat plant is an important endeavor.
The most intensive effects on plants was exerted by Cd, and then by Cu and Zn. Cadmium applied as treatment produce the least number of grains, the control plot produce grain yield more than the Cd treated plot (394 g). Cu and Zn produce more grains compared to the control (Ck) plot (Cu=361 g, Zn=387.5 g). The Heavy metals applied in combination had no effects on grains yield production. There were no significant differences between combination like Cu/Cd, Zn/Cu, Cd/Zn and Cd/Zn/Cu at p≤0.05. It was interesting to see that, Zn/Cu produce the highest grain yield (544 g) closely followed by Cd/Cu/Zn (533.25 g).
Accumulation of heavy metals in wheat plants
The ability for heavy metals accumulation in the shoots, grains and roots of durum wheat show a discrepancy, due to the different functions of different parts of wheat plant or the different natures of a number of HMs in wheat. In the midst of the studies plants parts, the three different irrigated solutions, with different treatments levels of Cd, Cu and Zn, had no significant effect on plant growth (Figure 10). The result of this study indicate that Cd and Zn were predominantly accumulated and distributed in wheat shoots and grains, and some proportion of these metal s remain in the roots. And this finding is in consistent [39]. In wheat plant, the grain is the edible parts that are consumed directly by humans. Therefore, high level of heavy metals in the grain need to be monitor as elevated level of these HMs endangers human health. The uptake of Cd in the grains was found to be very high in T2, T5 T6 and T8; meanwhile, it was very low at T1, T3 and T4. Whereas Cu uptake was noticed to be high in T3, T5, T7 and T8. Also, it was very low in T1, T2 and T6. For the uptake of Zn, T4 and T7 recorded the highest values of Zn uptake. The high level of Cd and Zn in this result is in consistent with a study conducted [40]. The Concentrations of Cd and Zn in grain were above the permissible limits of Cd and Zn in edible plants set by the FAO/WHO: Cd=1.95 and Zn=3.07 in the grains.
Figure 10: Effects of different treatments on the wheat grain yield. Ck: Soil/ mineral fertilizer, Cd: 8 mg, Zn: 25 mg, Cu: 300 mg. (Values are expressed as means±standard deviations of four replications after the green house trail). Bars with the same letters are not significantly different according to Duncan’s multiple range tests at p≤0.05
Thus, this result can be attributed to the high level concentration of these heavy metals in the soil. Therefore, the high levels of HMs concentration in soil need to be monitor as toxic uptake by plant is not generally safe for human consumption. Also, a Comparable result on Zn uptake was attained by Lavado [41]. Cd and Zn are the most accumulated heavy metal in all the three wheat plant parts (root, shoot and grain) as confirm in this study. On the other hand, Cd may promote the growth of wheat to some extent because there was no Cd stress observes during the plant growth period. For Zn levels at 25 mg/L, Cd had no effect on Zn uptake most likely because plant Zn uptake had reached its topmost. Also, it was observe that Zn in combination with Cd lower Cd uptake in this experiment, as also found by Reijnders [42,43]. On the other hand, mentioned that Zn in general improved the uptake of plant Cd. Zn uptake decreased when used in combination with Cd in the soil. This performance was also similar for the Cd 8/300 Cu concentrations found in the roots and in the shoots (Table 3). The grains accumulated more Cd8/25 Zn concentrations then the shoots and roots uptake of Cd and Zn in edible durum wheat grain). The combined application had no effect on the concentration of Zn and Cd uptake in the root, shoot and grain. The treatment where Zinc was added without Cd and Cu, registered the highest level of Zn concentration with the values of 3.07 and 2.98 mg/Kg for the grains respectively. Similar result was reach in a study conducted by Alaoui-Sossé with values ranging from 3.16 mg/kg Zn and 3.09 mg/kg [44]. The interactions of Cu and Zn can be observed in several different ways as it was reported: Zn strongly decreases Cu absorption, Cu is more successful to Zn absorption and Cu nutrition affects the redistribution of Zn within plants [45].
Correlation
Pearson correlation analysis was performed in order to investigate the relationships between DGT, HAc, CaCl2, HNO3 and Soil solution-extractable heavy metals, the total metal contents in the soils and the concentration of the heavy metals in different wheat plant parts. The Correlation analysis is a statistical method that establishes relationship between two elements. In wheat, grain is the direct human-edible part. Therefore, the heavy metal levels in grains affect human health. Therefore, the correlation of heavy metal concentrations in grains should be analyzed with heavy metal concentrations in the roots and shoots to determine the transfer relationship of these HMs in the plant grains.
Linear relationships between extractable heavy metals and plant uptake heavy metals concentrations measured by various extraction methods (DGT, HAc, HNO3, and CaCl2 and soil solution) were used to predict extractable heavy metals and heavy metals plant uptake in the various parts of wheat plant. However, single factor correlation analysis cannot evaluate the impact between extractable heavy metals concentrations and heavy metals uptake by plant. In this study, both simple linear regressions and stepwise multiple linear regressions (SMLRs) were applied to study the correlations between extractable heavy metals concentrations in the soil and heavy metals content in durum Wheat. (Table 4) shows the correlations between the total and DGT, HAc, CaCl2, HNO3 and Soil solution extractable heavy metal concentrations in the soils on one hand and the content of heavy metals in the root, shoot and grain of the wheat plants on the other hand.
The total metals content in the soils did not correlate significantly well with the concentration of the metals in the different wheat plant parts; except for Cu. In the same way, results attained from a study conducted by Alloway [46], confirmed that the total metal content in soils is a poor indicator of bioavailability in soil. Consequently, on the other hand found some strong correlations (p<0.001) between the soil and heavy metal concentrations in maize and wheat, but only for the micronutrients (Mn, Cu and Zn). Study on the accumulation of Heavy Metals in Different Parts of Wheat Plant confirm Significant correlations at (p<0.05) between EDTA extractable heavy metals and the concentrations of Cu, Zn and Ni in the roots of wheat. They further confirm that a positive correlation existed between the EDTA extractable concentrations of Cu and Zn in the soil and the Cu and Zn concentrations in the roots (r = 0.978* and r=0.983*) respectively [47]. The correlation between CaCl2 extractable Cu and Zn contents in the soil and the concentration of Cu and Zn in the wheat grains were high, but not significant at p<0.05(Table 5).
| Zn | Soil solution | CaCl2 | HAc | Roots | Shoots | Grains | HNO3 | DGT |
|---|---|---|---|---|---|---|---|---|
| Soil Solution | 1.000 | |||||||
| CaCl2 | 0.034 | 1.000 | ||||||
| HAc | 0.746*** | -0.039 | 1.000 | |||||
| Roots | 0.666*** | 0.052 | 0.788* | 1.000 | ||||
| Shoots | 0.348 | -0.018 | 0.521* | 0.740** | 1.000 | |||
| Grains | 0.573*** | 0.129 | 0.555* | 0.913* | 0.685*** | 1.000 | ||
| HNO3 | 0.588*** | 0.096 | 0.569* | 0.916* | 0.689*** | 0.990*** | 1.000 | |
| DGT | 0.515** | -0.022 | 0.413* | 0.246 | 0.052 | 0.135 | 0.169 | 1.000 |
***Correlation is significant at the 0.001 level; **Correlation is significant at the 0.01 level, the same below
Table 5: Correlations of Zn concentrations in durum wheat grains, roots, and shoots vs. extractable Zn by CaCl2, HAc, HNO3, Soil Solution and DGT
The diffusive gradients in thin films (DGT) measurement of Cu correlated well with the Cu plant uptake (R2=0.875***, p<0.001). The concentration of Cu extracted by DGT, HAc, CaCl2 and HNO3 significantly correlated to Cu plant uptake. The measurement of DGT show the strongest correlation (R2=0.875***, p<0.001). Copper is seems to be an element which was easy to assess the plant available concentration regardless of the use procedures. However, DGT show the strongest correlation for all the use methods use which was in consistent with previous study carry out by Anderson. He concluded that significant correlations exist between plant Cu uptake and measured Cu concentration by DGT (R2=0.64** and R2=0.86***). Mason conducted a research on metal accumulation in plant part (Wheat) and concluded that the DGT methods predicted Cu concentration significantly compare to the conventional extraction methods. They further said, the R2 values obtained (0.875**) indicates a very strong correlation and could be a promising result for DGT (Table-6).
| Cd | Soil Solution | CaCl2 | HAc | Roots | Shoots | Grains | HNO3 | DGT |
|---|---|---|---|---|---|---|---|---|
| Soil Solution | 1 | |||||||
| CaCl2 | 0.737*** | 1 | ||||||
| HAc | 0.643*** | 0.577*** | 1 | |||||
| Root | 0.456** | 0.476** | 0.917*** | 1 | ||||
| Shoot | 0.614*** | 0.673*** | 0.091 | 0.045 | 1 | |||
| Grain | 0.441* | 0.283 | 0.933*** | 0.901*** | -0.157 | 1 | ||
| HNO3 | 0.418* | 0.106 | 0.555*** | 0.328. | -0.109 | 0.622*** | 1 | |
| DGT | 0.471** | 0.225 | 0.895*** | 0.829*** | -0.137 | 0.963*** | 0.616*** | 1 |
***Correlation is significant at the 0.001 level; **Correlation is significant at the 0.01 level, the same below
Table 6: Correlations of Cd concentrations in wheat grain, root, and shoot vs. extractable Cd by CaCl2, HAc, HNO3, Soil Solution and DGT.
For this research project, the DGT method showed a significant correlation with HNO3 (R2=0.875***) and soil solution (R2=0.856***) extractable Cu. The measured Cu concentration by CaCl2 in this study showed an interesting correlation (R2=-0.461**), with a negative regression line for root Cu. All the extraction procedures correlated well, demonstrating that DGT is a good method for soil analysis. The aim with DGT in the research was to find a method that assessed plant available Cu, Zn and Cd better than the conventional methods; therefore, it can be discuss to what a good correlation means when using DGT with the conventional extraction methods.
On the other hand, HAc extractable Zn and HNO3 extractable Zn correlated significantly to Zn plant uptake (R2=0.788***, p<0.001) and (R2=0.990***, p<0.001). CaCl2 (R2=0.05, p=0.779) show no correlation between Zn plant uptake and extractable Zn. DGT measured Zn and HNO3 extracted Zn did not show any significant correlation with the plant uptake of Zn (R2=0.169, p=0.355). A report shows that the DGT measurement of Zn were strongly correlated to plant Zn uptake (R2=0.87). Andersson reported that DGTextractable Zn correlated well to extracted Zn plant uptake which was not established in this study. The measured Zn concentration by DGT showed an interesting correlation (R2=-0.022), with a negative regression line. It is however interesting that the relationship between DGT and CaCl2 correlated better than DGT and HNO3 for plant uptake. This indicated that the methods follow each other but cannot necessarily predict an accurate value of Zn. Ashraf reported that the DGT technique does not perform well in predicting Zn uptake by grass, lettuce, and lupine in terrestrial environments.
Zn is an element that is seems to be invariable, it is however difficult to assess the accurate amount or concentration of Zn taking up by plants. On the other hand, Zn is an element easy to become contaminated from various sources [48,49]. Therefore, the poor result obtained from this study may not certainly be the used methods.
Also, significant relationship was established between all of the extraction methods use for the soil analysis (HNO3, soil Solution, HAc and DGT) and plant uptake of Cd at (p<0.001). Several studies have concluded that the DGT technique is superior to traditional methods for assessing Cd bioavailability in different species of plants, including wheat, maize and ryegrass [50].
Soil characteristics like, soil heavy metal contents, pH, organic matter, and texture are commonly reported to influence the uptake of heavy metals by wheat. However, most of these previous studies were conducted by pot or field plot experiments, and were focused on the areas with special interest and associated with risk assessment like in this study. With this study, different heavy metals uptake by wheat was observed and their risked to plant and humans through its transferred to the food chain. Cadmium solution level had an effect on plant Cd uptake. Cadmium plant uptake was stimulated by higher plant growth and by a Zn level under 25 mg/l. Extracted Cd by DGT showed a strong significant correlation with extracted Cd from HAc, HNO3, and soil solution. Stronger correlation was establish between DGT and HAc (R2=0.895***) than DGT and CaCl2 (R2=0.225) in this study (Table 7).
| Cu | Soil Solution | CaCl2 | HAc | Roots | Shoots | Grains | HNO3 | DGT |
|---|---|---|---|---|---|---|---|---|
| Soil Solution | 1 | |||||||
| CaCl2 | -0.454** | 1 | ||||||
| HAc | 0.656*** | -0.369* | 1 | |||||
| Roots | 0.814*** | -0.461** | 0.751*** | 1 | ||||
| Shoots | 0.243 | -0.348 | 0.118 | 0.095 | 1 | |||
| Grains | 0.805*** | -0.212 | 0.619*** | 0.747*** | 0.062 | 1 | ||
| HNO3 | 0.860*** | -0.448* | 0.630*** | 0.863*** | 0.132 | 0.823*** | 1 | |
| DGT | 0.856*** | -0.466** | 0.599*** | 0.776*** | 0.143 | 0.780*** | 0.875*** | 1 |
Table 7: Correlations of Cu concentrations in wheat grain, root, and shoot vs. extractable Cu by CaCl2, HAc, HNO3, Soil Solution and DGT
This kind of result was expected considering the fact that CaCl2 is a weaker extraction method which extracted less plant-available metals than the stronger extraction methods. CaCl2-extractable fraction is considered to represent the soluble and easily exchangeable metals in the soil. In a study, Walker et al. found significant correlations between Cu, Zn and Mn concentrations in the shoots and the CaCl2-extractable concentrations of Cu, Zn and Mn in the soil. Most recently compared DGT with the traditional methods to assessed Cd bioavailability in soils. They reported that the Correlations between the plant and soil Cd concentrations measured with the traditional extraction techniques were dependent on the pH and organic carbon (OC) content, indicating that these methods are influenced by the soil properties.
Among the Plant part study, the roots have the strongest accumulation capacity for all the HMs, which are the parts in direct contact the with soil, moreover, the roots is the main channel of heavy metal uptake in wheat plant. In the midst of the three HMs study, Cd is the most accumulated heavy metal in all the three wheat plant parts (root, shoot and grain), and the Cu is the least accumulated heavy metal in all the plant parts. In different wheat plant parts, there are some correlations between the different HMs, but no consistent patterns are found, which shows that the enrichment process of different heavy metals in wheat is very complex. Of all the extraction procedures used in this study, DGT technique extracted more Heavy Metals compare to the soil solution, HAc and HNO3. It was established therefore in this study that DGT showed accurate concentration of plant available Cu than HNO3 but not of plant available Zn and Cd. Copper plant concentration showed significant correlation to extracted Cu by all the methods (DGT, HNO3, CaCl2, HAc and Soil Solution) but DGT had the strongest significant correlation at (R2=0.875***, p≤0.001).
Citation: Kolleh AD, Rong X, Bitondo D,et al. Evaluation of holistic approaches to predicting the concentrations of heavy metals in pots-cultivated durum wheat (Triticum durum L.). AGBIR. 2021;37(5):177-188.
Received: 08-Sep-2021 Accepted: 30-Sep-2021 Published: 07-Oct-2021
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
