Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2022) Volume 38, Issue 4
Glyphosate is one of the most widely used herbicides in Brazil and worldwide for controlling weeds in various agricultural crops, which raises concern on possible environmental impacts and human health. The present study aimed at assessing the induction of mutagenic changes and oxidative stress in different tissues of animals exposed to glyphosate and Trop®. Male Swiss mice were submitted to gavage treatment with glyphosate and Trop® at the concentrations of 50 and 200 mg/kg for 5 days. After the treatments the animals were euthanized and their bone marrow, liver, lung, and kidney were collected. The bone marrow was used for micronucleus. The liver, lung and kidney were submitted to biochemical analyzes of Thiobarbituric Acid Reactive Substances (TBARS), catalase enzyme, total antioxidant, in addition to evaluating the expression of Bax, Bcl2 and p53 proteins, as well as the expression of p53 gene transcription. The animals were weighed before and after the treatments. The results showed a significant weight reduction in the animals treated for 5 days. The occurrence of micronuclei was significant in the bone marrow. A significant increase in lipid peroxidation, besides changes in both, the catalase enzyme and total antioxidant levels were seen in all tissues. There was a decrease in p53 gene and Bax protein expression, and an increase of the Bcl2 protein expression. These results might suggest that the exposure to glyphosate and the consumption of antioxidant defenses can cause oxidative stress. In addition, changes in cellular biomarkers regarding glyphosate and Trop® were similar.
Glyphosate; Carcinogenic potential; Oxidative stress; Gene transcription
Considering the world scenario of a growing demand for food production and, thus, greater productivity, the use of mechanisms to enhance agricultural activities has become increasingly necessary [1]. Commercial herbicide-based formulations are the main resources used to reduce agricultural pests and supply the current food demand. Glyphosate (N-phosphonomethyl-glycine) is one of the most used herbicides in crops [2].
In Brazil, several commercial formulations are available for this herbicide, such as Roundup® by Monsanto and Trop® by Adama that include the active ingredient glyphosate and surfactant PolyethOxyEthyleneAmine (POEA) in their formulations, in addition to the Glyphosate® itself, that is, pure salt, by Fersol SA. These formulations are used for weeding control [3,4].
Glyphosate is the most used pesticide in Brazil and in the world, it had a significant increase in use after the development and release of transgenic crops resistant to this herbicide, with this there is evidence of persistence of glyphosate in food and water for human consumption [5-7].
In Brazil the glyphosate has been used since the late 1970s, however, it had a significant increase in the 2000s, according to data from the Brazilian Institute for the Environment and Renewable Natural Resources (IBAMA). According to an IBAMA report in 2019, glyphosate was the first in the ranking of the most sold herbicides in Brazil, with more than 217 thousand tons [8]. Global reports and studies on the use of glyphosate show that in Brazil the average consumption of the product is approximately 19 kilos per hectare [6,8].
In Brazil, the maximum allowed glyphosate limit is 10 mg/Kg and 500 ug/L in food and water respectively. When compared to the maximum limits allowed in food and water in the European Union, Brazil shows values 200 and 5000 times higher, respectively [5-7]. Therefore, conducting studies that assess the impact of exposure to this pesticide in the short term is increasingly relevant given the large consumption in Brazil and worldwide.
Glyphosate-based herbicides may be responsible for inducing the formation of reactive oxygen species, such as hydrogen peroxide (H2O2), superoxide anion (O2-), hydroxyl radical (OH) or other nitrogen species, which can cause imbalance between the pro-oxidant and antioxidant defense mechanisms, resulting in oxidative damage [9]. As a consequence of oxidative damage, lipid peroxidation indicates changes in the cell membrane, cohesion, flow, permeability, and metabolic function, which leads to cell instability with consequent cell injury and death [10,11].
After over 40 years of being globally used, glyphosate has been classified as ‘probably carcinogenic’ to humans by the International Agency for Research on Cancer (IARC). Such a reclassification of glyphosate by IARC was based on four studies that showed a high frequency of Non-Hodking Lymphoma (NHL) in glyphosate-exposed workers [12,13]. Although the induction mechanism of Non-Hodking Lymphoma through glyphosate has not been completely clarified, Bolognesi, et al. [14] showed slightly increased levels of micronuclei in glyphosate-exposed workers in Colombia.
Although IARC considered glyphosate as ‘probably carcinogenic' to humans, regulatory agencies worldwide do not share the same opinion, since they believe that the evidence of carcinogenesis induction by the oxidative stress mechanism is still not convincing [15]. Therefore, further studies that prove the involvement of oxidative stress with the appearance of DNA damage induced by glyphosate are needed. On the other hand, Kwiatkowska, et al. [16] carried out studies with human peripheral blood in 2017 that showed damage to leukocyte DNA, in addition to increased methylation of the tumor suppressor p53 gene. Based on this evidence, the present study aimed at assessing the correlation among oxidative stress, changes in the expression of proteins related to apoptosis Bax, Blc2 and p53, and the emergence of micronuclei in animals exposed to both, glyphosate, and the glyphosate-based formulation (Trop®).
Animal care
Healthy adult male Swiss albino mice (25-30 g) were housed in polyethylene cages under controlled laboratory conditions at 22 ± 2°C with a 12 h light/dark cycle maintained with lights on from 7 a.m. to 7 p.m. They were allowed 1-week acclimatization period before the onset of the experiment. All procedures were approved by the Institutional Ethics Committee of the Brazilian university referred to as Universidade do Oeste de Santa Catarina (UNOESC) (Opinion number: 003/2015). In all experiments the animals were managed according to the principles and guidelines for laboratory animal care.
Treatment
The animals were divided into six groups with 6 animals each. The doses of glyphosate and Trop® were chosen based on previous studies, in which the capacity of inducing oxidative stress in glyphosate-exposed animals was evaluated [17,18] and from the NOAEL (No Observed Adverse Effect Level) established by the ESFA 2015 and EPA 1993 which are 50 mg/kg/day and 500 mg/Kg/day respectively (EFSA [19,20]. Furthermore, recent works show that a dose of 200 mg/kg/day is capable of causing damage to the offspring of exposed Wistar rats [21]. The animals were submitted to gavage treatment for a period of 5 days. The 5-day period was chosen based on human exposure to this pesticide which normally occurs every 24 h 5 times a week. Besides, intended to verify whether glyphosate can promote important changes in a short period of exposure, since published works are always concerned with assessing damage after a long exposure [17,18]. The glyphosate by Sigma-Aldrich (N-Phosphonomethyl-glycine 96%) and the commercial glyphosate-based formulation Trop® by Milenia Agro Ciências S/A (Glyphosate 480 g/L) were used in the treatments. For experiments, glyphosate and Trop® were dissolved in sterile distilled water. All groups had free access to water and food during the experimental period. The groups were divided as follows: C (control): animals that were not submitted to treatment; CP: animals submitted to treatment with 0.2 ml of cyclophosphamide (via intraperitoneal) under a concentration of 50 mg/kg-only once-according to the methodology [22]. G50: animals submitted to oral treatment with glyphosate at a dose of 50 mg/kg; G200: animals submitted to oral treatment with glyphosate at a dose of 200 mg/kg; T50: animals submitted to oral treatment with Trop® at a dose of 50 mg/kg; T200: animals submitted to oral treatment with Trop® at a dose of 200 mg/kg. At the end of the treatment, the animals were weighed and euthanized by using an overdose of ketamine/xylazine. Bone marrow samples were collected for tests. The liver, lung and kidney samples were removed for assessing the oxidative stress, the expression of Bax, Bcl2 and p53 proteins, as well as the expression of p53 gene transcription.
Weight gain (WG)
The animals were weighed at the beginning and end of the treatments. The weight gain was determined by applying the following formula: WG=(final weight − initial weight).
Relative weight (RW) of the liver, lung and kidney
The organs were weighed after being immersed in sodium chloride 0.9% so that the excess of blood was removed; and gauze was used to remove the excess of liquid from the organs. The RW of the organs per g of animal was calculated based on the following formula: RW=(tissue weight ÷ body weight).
Evaluation of the oxidative stress in the liver, lung and kidney tissues
The tissues were quickly removed and homogenized (1:10, w/v) in a buffer containing 1% Triton X-100 150 mM, NaCl 20 mM sodium phosphate, pH 7.4. The tissues were homogenized in a tissue homogenizer for 30s on ice, followed by centrifugation at 10000 × g for 10 min. Lipid peroxidation was determined by measuring the Thiobarbituric Acid Reactive Substances (TBARS) level, which was defined according to the method already established by Bird et al. [23]. Catalase activity was determined following the methodology by Aebi [24]. Tissue antioxidant capacity was defined according to the Ferric Reducing Antioxidant Power (FRAP) assay as described by Benzie [25]. Under this procedure, the antioxidants of the tissue were evaluated as reducers of Fe3+ to Fe2+, which is chelated by 2,4,6-Tris (2-Pyridyl)-s-Triazine (TPTZ) to form a Fe2+ TPTZ complex with maximum absorbance at 593 nm. Tissue homogenates were mixed with of reagent containing 1.7 mM FeCl3 and 0.8 mM TPTZ, prepared in 300 mM sodium acetate (pH 3.6). The samples were incubated for 15 min at 37°C, and the absorbance was read at 593 nm (Bioplus BIO 2000, Barueri, SP, Brazil). The 6-hydroxy 2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was used as standard, and FRAP values were expressed as Trolox equivalents in micromoles per gram of tissue.
Analysis of the expression of Bax, Bcl2 and p53 proteins
The Western Blotting technique was used to determine the expression of Bax, Bcl2 and p53 proteins. Liver, lung, and kidney homogenates were prepared with 0.2 mM sucrose, 1 mM EDTA, 10 mM Tris and 1% protease inhibitor (Roche Diagnostics GmbH, Mannhein, Germany). The homogenates were incubated for 30 minutes at 4°C, centrifuged in 13,000 x g for 30 minutes at 4°C, and the supernatant and aliquot of the samples were removed. The proteins (50-100 µg) were separated with 12% polyacrylamide gel and electrically transferred to nitrocellulose membranes (Amershan ™ Protan ™ Premium 0.45 µm NC). Then, the membranes were placed in the Tris/buffered saline/Tween-20 blocking solution (TBS-T-5% skim milk powder in phosphate-saline-Tris buffer with 0.05% Tween-20) for one hour at room temperature (RT). After that the membranes were incubated overnight at 4°C with a monoclonal antibody clone, Bax (1:1000), Bcl2 (1: 1500) and p53 (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and β-actin (1:10000 Sigma, St. Louis, MO, USA), used to normalize the intensity of the bands among the samples. The membranes were washed with T-TBS and incubated for one hour at room temperature with a rabbit anti-immunoglobulin antibody, combined with HRP (Santa Cruz Biotechnology, INC). The proteins were detected with chemiluminescence by using a commercial ECL kit (Amersham Pharmacia Biotech, Uppsala, Sweden). The membrane was then inserted into a cassette along with the developing film (Amersham Hyperfilm ECL, UK) for approximately 10 minutes. After developing, the film was washed, dried, and scanned. The image program (Image J) was used to quantify the bands with densitometry. Protein content was determined according to the method by Lowry, et al. [26].
Analysis of the p53 gene transcription
The transcripts were determined by using the semi-quantitative RT-PCR method with adaptations by Chen, et al. [27]. The total DNA of the liver, lung and kidney samples was extracted from 100 mg of each tissue and 1 mL of Trizol® (Invitrogen™) according to the manufacturer's instructions. The extracted DNA was quantified with spectrophotometric reading and the purity was evaluated using the 260/280 and 260/230 ratios. Two micrograms of DNA were treated with DNAse (Invitrogen™) following the manufacturer's protocol. The synthesis of complementary DNA (cDNA) was performed by using the High-Capacityc DNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). The reaction was incubated at 25°C for 10 minutes, followed by 37°C for 2 h, and finally at 85°C for 5 minutes. Thus, a final cDNA volume of 20 μl was obtained, and stored at -20°C until its use for amplification reactions. The cDNA obtained from the samples was subjected to application analysis trough PCR (Polymerase Chain Reaction), using in the reaction 2 mM MgCl2, 1 mM of dNTPs (triphosphate deoxyribonucleotides), 1 U Taq polymerase (Invitrogen), 5% DMSO, 25 ρM of oligonucleotide Senso, 25 ρM of the antisense oligonucleotide specific for each gene shown in Table 1, PCR buffer (10 X) (Invitrogen), 1-3 µL of cDNA and ultrapure qsp water for 25 µl. The amplification reaction was performed with denaturation at 94°C (5 minutes), followed by 25-35 cycles of 94°C (2 minutes), 55-58°C (1 minute), 72°C (2 minutes) and final extension at 72°C (10 minutes), after optimizing the reaction for greater efficiency of semi-quantitative analysis [28].
| Genes | Sense | Antisense | Product (pb) |
|---|---|---|---|
| ratp53 | 5´-ACAGCGTGGTGGTACCGTAT-3´ | 5´- GGAGCTGTTGCACATGTACT-3´ | 83 |
| GAPDH | 5´- GTATTGGGCGCCTGGTCACC-3´ | 5´- CGCTCCTGGAAGATGGTGATGG-3´ | 202 |
Table 1: Oligonucleotides used in the RT-PCR reaction.
The amplification results were evaluated through electrophoresis at 1% agarose gel with 0.5 μg/mL of ethidium bromide for 2 hours at 3 VCM-1. After electrophoresis, the DNA was visualized under ultraviolet light with short wavelength, coupled to a documentary photo that recorded the image and sent it to the computer; a digital image was captured to read the amplified DNA profiles. A 100 base pair molecular weight marker (Ludwig Biotec) was used as standard. The ratio between the densitometry of the amplification product per RT-PCR of the p53 and GAPDH genes was analyzed by using the PhtoCaptVersion 12.4 program (Vilber Lourmat), which generated the values plotted in graphs (Table 1) Source: [28,29].
Bone marrow micronucleus test
The micronucleus test in bone marrow was carried out according to the method described by Eriksson, et al. [30]. The distal epiphyses of the femurs were cut; the bone marrow (BM) was removed with a syringe containing 1 mL of fetal bovine serum and deposited in a centrifuge tube with an additional 1 mL of bovine serum. The material was homogenized and centrifuged for 10 minutes at 145 × g; a part of the supernatant (approximately 1.7 mL) was discarded and the pellet was resuspended in the rest of the supernatant. To prepare the slides, a drop of BM suspension was placed on one end of a clean dry glass slide, making an extension with the aid of a glass coverslip, and then the slides were air-dried. After 24 hours the slides were fixed in absolute methanol for 10 minutes and air dried. After 24 hours of fixation, the slides were stained with Giemsa (Newprov) diluted in Sorensen buffer (0.06 M Na2HPO4 and 0.06 M KH2PO4-pH 6.8) and washed in distilled water, air dried, and then stored in the refrigerator (4°C) until cytological analysis. For each animal, 1000 polychromatic erythrocytes (PCEs) and 1000 Normo Chromatic Erythrocytes (NCEs) were analyzed and the result expressed in total of cells containing micronuclei. The PCE/NCE ratio was obtained by counting 1000 erythrocytes. The slides were analyzed by using a visible light microscope with an immersion objective of 100x and an ocular lens of 10x; a final increase of 1000. The MN counting was performed according to the criteria described by Schmid and Heddle, et al. [31,32] and only the whole cells with a rounded intact cytoplasm shape were considered for counting. The intra cytoplasmic structures identified as micronuclei must be rounded and at the same plane as the cytoplasm, besides not having refringence.
Statistical analysis
The GraphPadPrism version 5.0 for Windows program (GraphPadSoftware) was used for the statistical analysis. Initially, a spreadsheet of the data was elaborated and the Descriptive Statistics was processed, which provided the mean values (M) and mean standard deviation (SD) to express the results. The analysis of variance (ANOVA) was initially used to compare three or more groups. When significant differences in ANOVA occurred, Tukey's multiple comparison test was used (comparing all groups against each other without fixing the control). The differences seen during the analysis were considered statistically significant when the probability was less than 0.05 (5%).
Effect of glyphosate or Trop® administration on the body and organ weight of Swiss mice
By assessing the results shown in Figure 1, it is seen that all groups of animals treated over this period had a significant weight decrease compared to the control (C) (p<0.05). The treatment with glyphosate 200 mg/kg showed a reduction of 2.10% in relation to the initial treatment weight, whereas the control group obtained a gain of 7.14% in the same period. When comparing the different treatments to each other, no significant difference was observed by the Tukey's test (Figure 1).
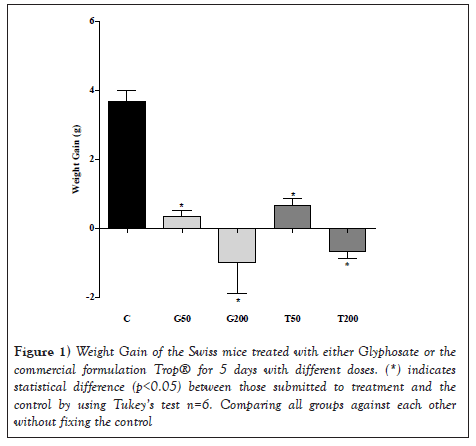
Figure 1: Weight Gain of the Swiss mice treated with either Glyphosate or the commercial formulation Trop® for 5 days with different doses. (*) indicates statistical difference (p<0.05) between those submitted to treatment and the control by using Tukey’s test n=6. Comparing all groups against each other without fixing the control
Considering the groups of animals treated and the control, organ-to-body weight ratio showed no significant result (Table 2). In addition, macroscopic analysis showed no difference in color, shape, or texture of the organs after euthanasia and removal of the animals' organs.
| Groups | Period of treatment | The animals’ organ-to-body weight ratio | ||
|---|---|---|---|---|
| Liver | Lung | Kidney | ||
| C | 5 days | 0.0460 ± 0.0041 | 0.0059 ± 0.0009 | 0.0143 ± 0.0006 |
| G50 | 5 days | 0.0470 ± 0.0042 | 0.0059 ± 0.0002 | 0.0140 ± 0.0015 |
| G200 | 5 days | 0.0470 ± 0.0062 | 0.0067 ± 0.0009 | 0.0144 ± 0.0022 |
| T50 | 5 days | 0.0441 ± 0.0075 | 0.0053 ± 0.0004 | 0.0134 ± 0.0029 |
| T200 | 5 days | 0.0520 ± 0.0049 | 0.0060 ± 0.0011 | 0.0122 ± 0.0031 |
Note: Results of the organ-to-boy weight ratio of control and treated animals for a number of six animals per group shown as mean ± standard deviation. Tukey’s tests showed no statistical difference between the animals submitted to the treatments and the control or with each other (p<0.05) n=6.
Table 2: Organ weight and final weight ratio of Swiss mice treated with glyphosate or the commercial formulation Trop® for 5 days with different doses.
Effect of the administration of glyphosate or Trop® on the induced oxidative stress in Swiss mice
Figure 2 shows the results related to oxidative stress markers in the liver (Figures 2A), lung (Figures 2B) and renal tissues (Figures 2C). There was a significant increase in the levels of lipoperoxidation assessed by applying the TBARS method and a decrease in the activity of the catalase enzyme in the liver tissue of the animals treated with 50 and 200 mg/kg weight of glyphosate, whereas the animals treated with Trop® did not show a significant difference in these markers. On the other hand, the total antioxidant levels showed a small reduction only in the treatment with 50 mg/kg of glyphosate. Considering the lung tissue, a significant increase in the levels of lipoperoxidation was seen in all treatments when compared to the control group, associated with a significant reduction in the levels of total antioxidant. Regarding the renal tissue, a significant increase in the levels of lipoperoxidation was seen in all treatments with a reduction in catalase activity; the levels of total antioxidant showed a reduction only in the treatment with 200 mg/kg of glyphosate or Trop® when compared to control animals.
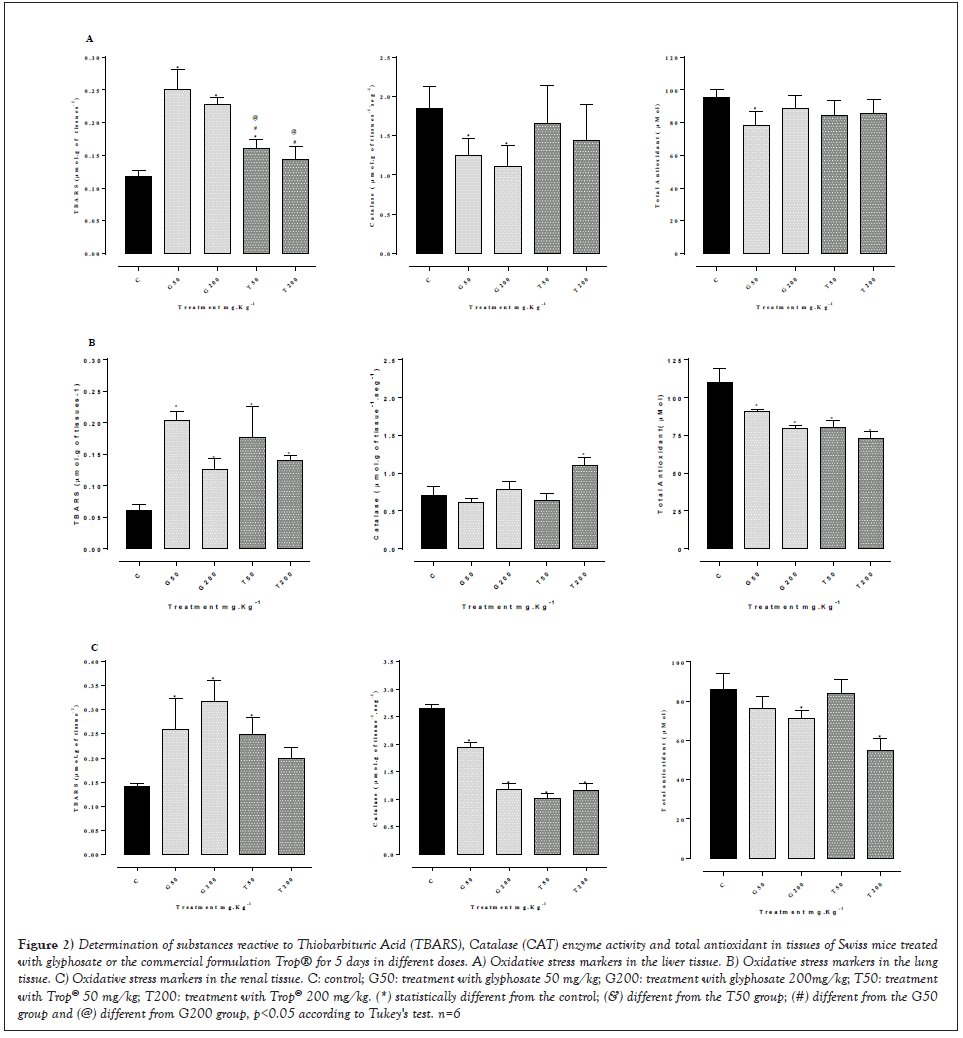
Figure 2: Determination of substances reactive to Thiobarbituric Acid (TBARS), Catalase (CAT) enzyme activity and total antioxidant in tissues of Swiss mice treated with glyphosate or the commercial formulation Trop® for 5 days in different doses. A) Oxidative stress markers in the liver tissue. B) Oxidative stress markers in the lung tissue. C) Oxidative stress markers in the renal tissue. C: control; G50: treatment with glyphosate 50 mg/kg; G200: treatment with glyphosate 200mg/kg; T50: treatment with Trop® 50 mg/kg; T200: treatment with Trop® 200 mg/kg. (*) statistically different from the control; (&) different from the T50 group; (#) different from the G50 group and (@) different from G200 group, p<0.05 according to Tukey's test. n=6.
Analysis of the expression of Bax, Bcl2 and p53 proteins
Figure 3 shows the expression of Bax, Bcl2 and p53 proteins in the liver (Figures 3A-3C) in animals treated with glyphosate or Trop® for 5 days. The analysis showed no significant difference in the expression of p53 protein in all tissues with any of the doses administered of glyphosate or Trop® when compared of control group, but when compared between the groups observed a significant in glyphosate groups. A significant reduction of Bax protein was seen in the liver, associated with a significant increase of Bcl2 in groups treat with 200 mg/kg. Considering the lung and renal tissues, the Bax protein did not change in relation to the control group, whereas Bcl2 showed a significant increase.
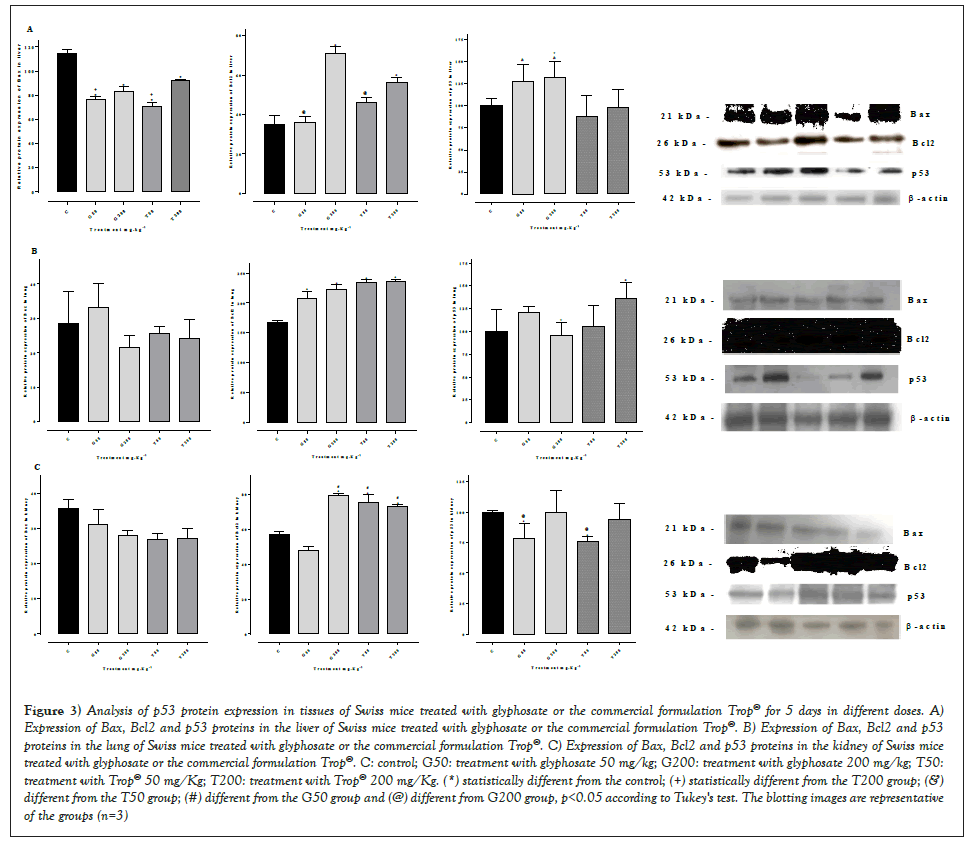
Figure 3: Analysis of p53 protein expression in tissues of Swiss mice treated with glyphosate or the commercial formulation Trop® for 5 days in different doses. A) Expression of Bax, Bcl2 and p53 proteins in the liver of Swiss mice treated with glyphosate or the commercial formulation Trop®. B) Expression of Bax, Bcl2 and p53 proteins in the lung of Swiss mice treated with glyphosate or the commercial formulation Trop®. C) Expression of Bax, Bcl2 and p53 proteins in the kidney of Swiss mice treated with glyphosate or the commercial formulation Trop®. C: control; G50: treatment with glyphosate 50 mg/kg; G200: treatment with glyphosate 200 mg/kg; T50: treatment with Trop® 50 mg/Kg; T200: treatment with Trop® 200 mg/Kg. (*) statistically different from the control; (+) statistically different from the T200 group; (&) different from the T50 group; (#) different from the G50 group and (@) different from G200 group, p<0.05 according to Tukey's test. The blotting images are representative of the groups (n=3).
In order to verify whether an inverse correlation between pro-apoptotic Bax and anti-apoptotic Bcl2 proteins occurred, the mean relative value of Bcl2 expression was divided by the mean relative value of Bax, which are shown in Table 3. An inverse correlation between Bcl2 and Bax occurred in the liver only at the highest doses (200 mg/kg), whereas in all treatments an inverse correlation in the lung was seen when compared to the control group. Regarding the kidney, the correlation was also shown to be inverse in groups G200, T50 and T200 (Table 3).
| Tissue | Groups | ||||
|---|---|---|---|---|---|
| C | G50 | G200 | T50 | T200 | |
| Liver | 0.306 | 0.4705 | 0.905 | 0.697 | 1.06 |
| Lung | 3.41 | 7.796 | 10.7 | 9.21 | 10.74 |
| Kidney | 1.6 | 1.55 | 2.83 | 2.81 | 2.70 |
Note: C: control; G50: treatment with glyphosate 50 mg/kg; G200: treatment with glyphosate 200 mg/kg; T50: treatment with Trop® 50 mg/Kg; T200: treatment with Trop® 200 mg/Kg.
Table 3: Correlation between Bcl2 and Bax proteins (Mean relative value of Bcl2 expression/Mean relative value of Bax expression) in tissues of Swiss mice treated with glyphosate or the commercial formulation Trop® for 5 days in different doses.
Analysis of p53 gene transcription
The gene expression analysis was performed through the relative quantification of each gene regarding a constitutive expression gene. The densitometry analysis of the bands of RT-PCR products, Figure 4 showed a reduction in the expression of the p53 gene transcripts in the liver (Figure 4A) with all doses tested, as well as in the lung (Figure 4B) in relation to the control. A decreased gene expression was seen (Figure 4C) in the renal tissue with the doses of Trop® 50 mg/Kg and Trop® 200 mg/Kg compared to the control, whereas the glyphosate-treated animals with the doses of 50 mg/Kg and 200 mg/Kg had no change in relation to the control group.
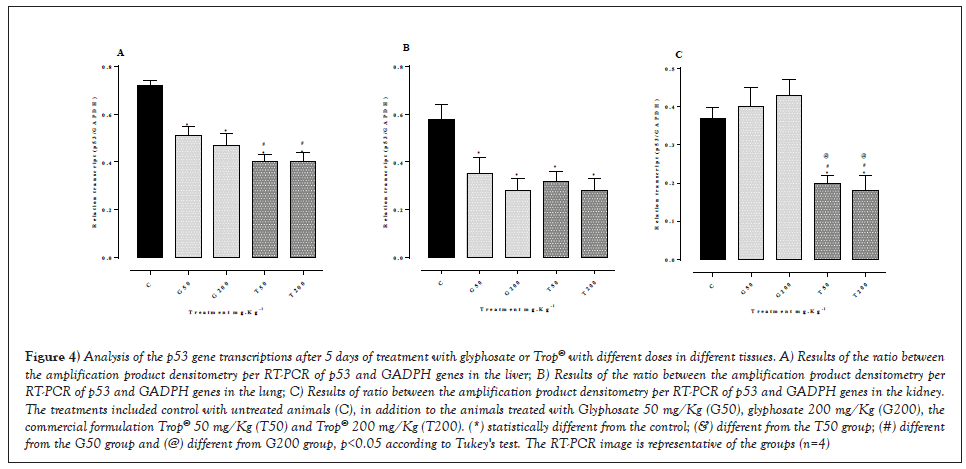
Figure 4: Analysis of the p53 gene transcriptions after 5 days of treatment with glyphosate or Trop® with different doses in different tissues. A) Results of the ratio between the amplification product densitometry per RT-PCR of p53 and GADPH genes in the liver; B) Results of the ratio between the amplification product densitometry per RT-PCR of p53 and GADPH genes in the lung; C) Results of ratio between the amplification product densitometry per RT-PCR of p53 and GADPH genes in the kidney. The treatments included control with untreated animals (C), in addition to the animals treated with Glyphosate 50 mg/Kg (G50), glyphosate 200 mg/Kg (G200), the commercial formulation Trop® 50 mg/Kg (T50) and Trop® 200 mg/Kg (T200). (*) statistically different from the control; (&) different from the T50 group; (#) different from the G50 group and (@) different from G200 group, p<0.05 according to Tukey's test. The RT-PCR image is representative of the groups (n=4).
Effect of the administration of glyphosate or Trop® on the emergence of micronuclei in bone marrow (BM) erythrocytes of Swiss mice
The statistical analysis of the results (Figure 5) highlighted that the frequency of polychromatic and normochromatic micronuclei in the BM erythrocytes showed a difference (p<0.05) in relation to the control. When comparing the frequency of micronucleated polychromatic findings and the micronucleated normochromatic ones of the treatments with glyphosate 200 mg/kg or Trop® 200 mg/kg, a statistical difference from the control was seen. The number of micronuclei in polychromatic erythrocytes for the glyphosate 50 mg/kg group and the Trop® 50 mg/kg one was within the normal range, characterizing the absence of mutagenic activity at this dose. Table 4 summarizes the results of the mean ± standard deviation of the number of erythrocytes with micronuclei, as well as the ratio between the number of polychromatic (MNPCEs) and normochromatic (MNNCEs) cells in the bone marrow of the mice treated with glyphosate or the commercial formulation Trop® and control groups.
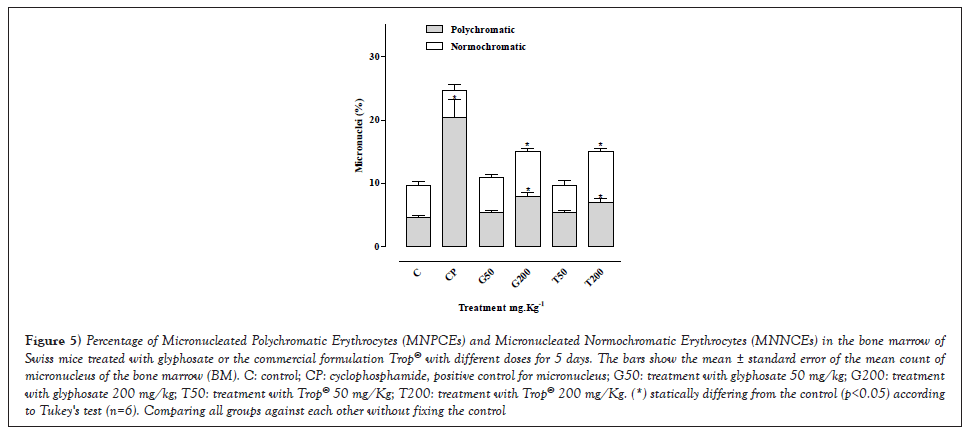
Figure 5: Percentage of Micronucleated Polychromatic Erythrocytes (MNPCEs) and Micronucleated Normochromatic Erythrocytes (MNNCEs) in the bone marrow of Swiss mice treated with glyphosate or the commercial formulation Trop® with different doses for 5 days. The bars show the mean ± standard error of the mean count of micronucleus of the bone marrow (BM). C: control; CP: cyclophosphamide, positive control for micronucleus; G50: treatment with glyphosate 50 mg/kg; G200: treatment with glyphosate 200 mg/kg; T50: treatment with Trop® 50 mg/Kg; T200: treatment with Trop® 200 mg/Kg. (*) statically differing from the control (p<0.05) according to Tukey's test (n=6). Comparing all groups against each other without fixing the control.
| Groups | Period of treatment | Number of micronucleated cells | ||
|---|---|---|---|---|
| MNPCEs | MNNCEs | PCE/NCE | ||
| CP | 1 day | 20.33 ± 2.90 | 4.33 ± 0.88 | 0.82 ± 0.13a |
| C | 5 days | 4.66 ± 0.33 | 5.00 ± 0.57 | 0.48 ± 0.12b* |
| G50 | 5 days | 5.33 ± 0.33 | 5.66 ± 0.33 | 0.48 ± 0.04b* |
| G200 | 5 days | 8.00 ± 0.57 | 7.00 ± 0.57 | 0.53 ± 0.04b* |
| T50 | 5 days | 5.33 ± 0.33 | 4.33 ± 0.88 | 0.55 ± 0.05b* |
| T200 | 5 days | 7.00 ± 0.57 | 8.00 ± 0.57 | 0.46 ± 0.02b* |
Note: 1000 PolyChromatic Erythrocytes (PCE) and 1000 normochromatic erythrocytes (NCE) were analyzed for each animal. (*) Statistically different from CP: cyclophosphamide control (p<0.05) according to Dunnet’s test. A letter alone differs statistically from the other p<0.05 according to Tukey’s test, whereas equal letters do not differ (p>0.05) n=6.
Table 4: Mean ± standard deviation of the number of micronucleated polychromatic erythrocytes (MNPCEs), micronucleated normochromatic erythrocytes (MNNCEs) and the PCE/NCE ratio in bone marrow cells of Swiss mice treated with glyphosate or the commercial formulation Trop® with different doses for 5 days.
Regarding the cytotoxic effects on the bone marrow, shown with the PCE/NCE ratio analysis, it is seen that no statistical difference occurred at the concentrations used in the treatments in relation to the control. The PCE/NCE ratio that indicates cytotoxicity was obtained with the ratio between PCE/(PCE+NCE) counted in 1000 cells.
The present study showed that oral exposure of mice to glyphosate or the commercial formulation (Trop®) based on glyphosate caused a significant reduction in body weight without changing the organ weight/animal weight ratio. Although the weight of such organs had not been affected, there was a significant change in oxidative stress markers in the liver, lung and kidney, a factor that may be associated with changes in the p53 gene, as well as in the expression of Bax, Blc2 and p53 proteins in these tissues [33], when administering a dose of the herbicide Roundap® corresponding to 500 mg/kg of body weight, noticed a significant weight reduction of approximately 10% in relation to the initial weight over 15 days of treatment. Studies report that the oral ingestion of glyphosate with surfactant can induce severe gastrointestinal injuries, which can be responsible for the reduction in food intake, thus, the weight reduction seen in animals exposed to glyphosate [34] is likely to be due to the change in palatability [35].
Food or water intake was not assessed in the present study, but only the weight of the animals, however, this may be an explanation for a significant reduction in the weight of the animals, which was dose dependent and higher in the group treated with glyphosate 200 mg/kg. Therefore, it can be inferred that weight loss is directly correlated to the active glyphosate principle rather than the surfactant found in the formulation.
Determining the weight of organs such as the liver, the kidney and the lung can provide indications of each organ function and the action of the substance tested on the organism [18] showed that the exposure of Swiss mice to low doses of glyphosate for 30 days cause changes in the mass of the brain, heart, lung, kidney, adipose tissue, or gall bladder. This effect was not seen in the present study about the liver, lung, and kidney weight (Table 2). However, a significant loss in body weight (Figure 1) occurred. This might have happened due to the short time of herbicide exposure, that is, only 5 days.
Figure 2 shows an increase in oxidative stress caused by the exposure to both glyphosate and the commercial formulation (Trop®) based on glyphosate. Oxidative stress is more evident in the pulmonary and renal tissues, since there was an increase in lipid peroxidation associated with a reduction in catalase enzyme activity and in total antioxidant defenses. Similar results were seen in the liver of mice treated with a single dose of either glyphosate or Trop® [36,37] and in fish exposed to the herbicide Roundup® [38] for 96 hours, as well as after maternal exposure to glyphosate-Roundup® with an increase in the levels of lipid peroxidation (TBARS) in the liver tissue of the babies of herbicide-exposed mothers [39].
The treatment with either glyphosate or Trop® for 5 days reinforces the hypothesis of antioxidant defense consumption, that is, when the production of reactive species surpasses the antioxidant defense capacity, the phenomenon of oxidative stress starts, resulting in morphological and functional disturbance of the injured cell, which can trigger molecular changes, such as the appearance of micronucleated cells, which shows a genotoxic effect (Figure 6). These data may suggest that either glyphosate or commercial formulations based on glyphosate can induce cellular changes capable of promoting different types of cancer. In this sense, an epidemiological study by Roos, et al. [12] showed a high incidence of multiple myeloma in glyphosate-exposed individuals.
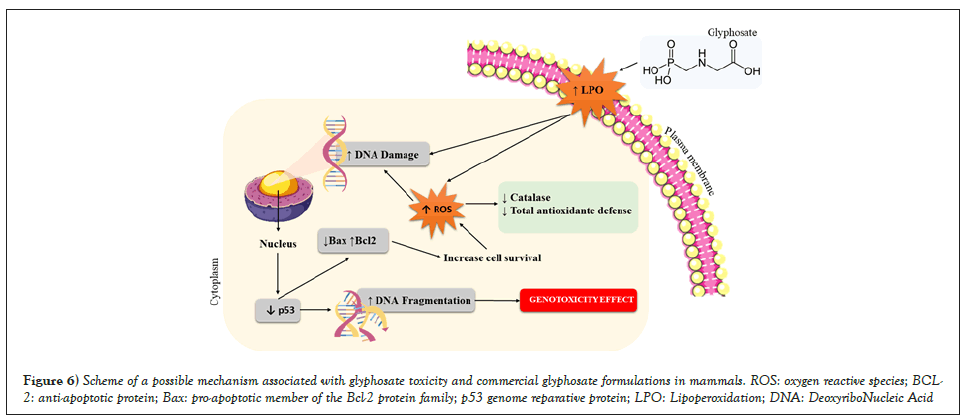
Figure 6: Scheme of a possible mechanism associated with glyphosate toxicity and commercial glyphosate formulations in mammals. ROS: oxygen reactive species; BCL-2: anti-apoptotic protein; Bax: pro-apoptotic member of the Bcl-2 protein family; p53 genome reparative protein; LPO: Lipoperoxidation; DNA: DeoxyriboNucleic Acid.
According to Zancanaro, et al. and Zhang, et al. [37,38] evaluations on genotoxicity and oxidative stress in aquatic planarians (Dugesia schubarti) exposed to glyphosate formulations, that is, Roundup® Original, Roundup® Transorb and Roundup® ready, showed that all formulations induced DNA damage and oxidative stress. The activity of SuperOxide Dismutase (SOD) increased between 4 and 16 hours of treatment, whereas TBARS levels decreased between 4 and 16 hours. Regarding the three formulations tested, CAT activity remained decreased at all periods of exposure. These results showed that glyphosate–based herbicide formulations can cause damage to DNA, in addition to changing the oxidative balance in planaria, which indicates that this herbicide toxicity is not restricted to plants.
Studies have reinforced the hypothesis that increased expression of the p53 protein can normally prevent the transformation process, whereas the decrease in p53 expression predisposes the cell to change [39,40]. In cells that have the mutated p53 gene and inactivation of the p53 protein, the cell cycle does not occur, which is necessary for DNA repair. These genetically unstable cells tend to accumulate additional chromosomal mutations and rearrangements, which leads to a rapid proliferation of cell clones with mutated DNA and neoplastic transformation [41]. Therefore, chemical substances capable of modifying the expression of p53 may be responsible for the appearance of cell lesions that culminate in the emergence of tumors.
The results of the present study showed a correlation among a reduction in the p53 gene, a reduction in Bax expression, and an increase in Bcl2 with oxidative stress. Figures 2-4 shows that a greater reduction in the p53 gene, liver and lung occurred in all treatments, and in the kidney submitted to the treatments with Trop®, which implied in a significant reduced Bax and increased Bcl2 associated with more pronounced oxidative stress. This is likely to have been triggered due to changes in the expression of p53, which is considered a sensor of cellular stress. The induction of oxidative stress is, thus, usually accompanied by the activation of p53, which works as a pro-oxidant factor to promote toxicities mediated by oxidative stress, favoring the death of mutated cells [42,43]. This was rather reported in renal cells treated with cisplatin, which showed an inhibition of p53 reduced oxidative stress and, thus, a decreased cisplatin-induced cytotoxicity [44].
The present study showed a reduction of the p53 gene that did not significantly reduce oxidative stress, resulting in less induction of apoptosis and greater cell survival, an effect evidenced by the reduction in Bax expression and increase in Bcl2 expression in lung, liver and kidney. Table 3 shows an inverse correlation between Bax and Bcl2, since the ratio between the two proteins was higher than that of the control group, especially in the pulmonary and renal tissues whose antioxidant defenses are lower. It can be inferred that there was an overexpression of Bcl2 and a reduction in Bax expression, which might reduce the apoptotic index and favor the proliferation of modified cells. Studies show that the higher the Bcl2/Bax ratio, the greater the cell survival and the appearance of mutations such as those that occur in cases of chronic lymphocytic leukemia or in lung tumors Velez, et al. and Liu, et al. [45,46].
On the other hand, Yuan, et al. [47] found an increase in Bax associated with a reduction in Bcl2 in the ovaries of mice treated with glyphosate. These differences in the data found by Velez, et al. [45] and the present study may be associated with the administered dose and the evaluated tissue. In the present study, was used a dose of 50 and 200 mg/kg, while [46] used a dose of 250 and 500 mg/kg in a slightly longer treatment time (7 days).
Yuan, et al. [47] reported that substances capable of up-regulating the expression of pro-apoptotic proteins (p53 and Bax) and down-regulating the expression of anti-apoptotic proteins (Bcl2) reduce the appearance of mutations that may cause the emergence of micronuclei. However, substances that have an opposite effect, as shown in the present study, can also favor the appearance of micronuclei, and can be considered pre-carcinogenic substances.
Table 3 shows a significant increase in Bcl2/Bax ratio in the lung and kidney when submitting the animals to treatment with glyphosate or the commercial formulation based on glyphosate. This may have favored the emergence of the genotoxic effect visualized in the bone marrow with the presence of micronucleated cells.
In this sense, the results of the present study suggest that oral exposure to either glyphosate or the commercial formulation (Trop®) based on glyphosate can induce cell damage by inhibiting the p53 gene, which results in greater oxidative stress and, thus, the emergence of the genotoxic effect visualized by increased micronucleated cells in the bone marrow. Similar results were obtained with the exposure of glial cells to the monocrotophic organophosphate [48] and after the exposure of fish to the organophosphate phorate [49]. The genotoxic effect shown in the present study may be associated with an increase of the Bcl2/Bax ratio, especially in the lung and kidney, (Table 3) Júnior, et al. and Velez, et al. [44,45] reported that the greater this ratio the greater the survival of mutated cells.
The present study showed a significant increase in the micronuclei of the bone marrow erythrocytes when exposing the animals to either glyphosate or Trop® at a dose of 200 mg/kg, which corroborates with the results by [35], thus, indicating a genotoxic effect of this herbicide in mammals.
It is worth mentioning that Zhang, et al. [50] proved the genotoxic activity by testing the micronucleus in peripheral blood cells of individuals exposed to agrochemicals, such as fungicides, insecticides, and herbicides. Another study by Ikumawoyi, et al. [51] showed that glyphosate, one of the herbicides most used by farmers, produces genotoxic damage in erythrocytes and gills of the fish Prochilodus lineatus [28] showed the genotoxic potential of pesticides used in soybean fields in view of the significant increase in cells with micronuclei in herbicide-exposed workers.
The present study showed the occurrence of a significantly higher micronucleus in groups of animals treated with higher doses, which indicates that the genotoxic action may be dose dependent, characterizing the presence of mutagenic activity in these doses, like those found in animals treated with cyclophosphamide, a drug known as being cytotoxic and genotoxic. 58 Compounds that induce the appearance of a genotoxic effect may be responsible for the emergence of tumors since this effect is related to changes in the tumor suppressor gene p53 [52-59].
In conclusion the results obtained in the present study were summarized in Figures. Exposure to either the glyphosate herbicide or commercial formulations based on glyphosate induce genotoxicity mediated by an increased oxidative stress caused by the inhibition of the p53 gene, reduction in expression of the pro-apoptotic protein Bax, and increased expression of the anti-apoptotic protein Bcl2, which might exert a pro-oxidant effect after exposure to the herbicide. This is a dose-dependent effect that can be evidenced in short exposures. However, further studies that evaluate the pro-oxidant involvement of p53, as well as Bax and Bcl2 proteins regarding the exposure to glyphosate and the mechanisms associated with it must be carried out in herbicide-exposed workers so that the genotoxic effects and their possible correlation with the increase in the number of the cases of cancer can be proven in humans.
This work was supported by CNPq (National Council for Research and Development) and UNIARP Research Support Fund (FAP).
The authors have no conflict of interest to report.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Zancanaro V, Bellaver EH, Baratto CM , et al. Glyphosate-induced oxidative stress, genotoxic effect and differential expression of p53, Bax and Bcl2 in different mice tissues. AGBIR. 2022; 38(4):305-313.
Received: 01-Jun-2022, Manuscript No. AGBIR-22-65535; , Pre QC No. AGBIR-22-65535 (PQ); Editor assigned: 06-Jun-2022, Pre QC No. AGBIR-22-65535 (PQ); Reviewed: 20-Jun-2022, QC No. AGBIR-22-65535; Revised: 27-Jun-2022, Manuscript No. AGBIR-22-65535 (R); Published: 05-Jul-2022, DOI: 10.35248/0970-1907.22.38.305-313
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
