Agricultural and Biological Research
RNI # 24/103/2012-R1
Review Article - (2024) Volume 40, Issue 3
Aquatic environments are recognized as the ultimate setting for the attainment and distribution of antibiotic resistance. Exposure of animals and humans to Antibiotic-Resistant Bacteria (ARB) and Antibiotic-Resistant Gene (ARGs) in environmental compartments may cause a severe health hazard. The Quantitative Microbial Risk Assessment (QMRA) has been recommended as an appropriate method to examine and measure the associated health hazard. Nevertheless, the specific information related to exposure and dose-response associated with ARB and ARGs in distinctive water usage circumstances required to perform a QMRA is very limited. The review reiterates the published research studies regarding the health risk assessment associated with ARB and ARG in the environment, and the challenges of tracing source of ARGs in the environmental compartments. It also summarizes the information related to sources, proliferation, dissemination, and management strategy for reducing the release of ARB and ARGs in aquatic environments. Studies on these topics will impart knowledge on more meticulous management options of antibiotic resistance and its distribution in aquatic environments and hazards associated with human health.
Human health risk; Antibiotic Resistance Genes (ARGs); Quantitative Microbial Risk Assessment (QMRA); Source tracing; Aquatic environments; Management strategy
Antibiotic resistance (AMR) has evolved as a global public health concern, rendering common infections untreatable. However, identifying and prioritizing operative control approaches for antibiotic resistance are in their early stages as the emergence and dispersal of Antibiotic Resistance Genes (ARGs) in several sceneries remain misconstrued [1]. Presently, there is a growing apprehension that the environment potentially contributes to ARGs growth beyond the clinical setting [2]. ARGs have long been evolved as an emerging contaminant, which are not usually tracked in the environmental compartments and can potentially cause adverse environmental and human health impacts [3,4]. Unlike other chemical compounds, ARGs behaviors in the environment are frequently connected with their biological aspects. It can be additionally obscured by physical, chemical, bio geographical and environmental factors. ARGs as environmental contaminants have not been comprehensively understood until the early 21st century [5]. More obvious positive associations between anthropogenic actions and environmental ARGs contamination were recognized. ARGs amount proliferates within closed proximity to human ecosystems as either point (pharmaceutical manufacturing and Wastewater Treatment Plants (WWTPs)) or diffuse (impacted water or sediment, and animal husbandry) sources [6]. Thus, categorizing potential sources and interpreting their associations with ARGs manifestation becomes critical in regulating ARGs propagation in the environment [7].
Aquatic environments are identified as the pools and dissemination pathways for propagating antibiotic resistance. Wastewater and drinking water treatment procedures are seemed to be vulnerable of completely eliminating ARGs. WWTPs effluents comprising ARB and ARGs can culminate in aquatic environments including lakes and rivers. Using treated wastewater for favorable aspiration like recreational and agricultural pursuits can instigate ARB and ARGs to the specific environment. Therefore, besides drinking, humans can be introduced to ARB and ARGs through recreational activities, agriculture, and ingestion of food produced with treated water. Though, prospective human health hazards linked with ARB and ARGs exist in aquatic environmental compartments have not been wholly assessed yet. The precise information related to exposure and doseresponse evaluation of ARB and ARGs in diverse water usage settings required to perform a Quantitative Microbial Risk Assessment (QMRA) is very limited. Apprehensions over the human health risks of antibiotic resistance related with antibiotic determinants in the environmental compartments are the latent hazard of consumed antibiotic residues varying human micro biome. The latent hazard of generating a selection pressure on the environmental microbial communities leads to reservoirs of ARB and ARGs and ARB is described to as environmental antibiotic resistance [8]. As antibiotics are broadly used now, human risk assessments linked with antibiotic resistance have drawn cumulative consideration in the environment. Health risk assessment estimates the probability of infection and deaths produced by the infection related with ARB, including four central components: hazard identification, exposure assessment, doseresponse assessment, and risk characterization. The study's primary objective is to describe a comprehensive view on health risk assessment of ARB and ARG and challenges of tracking origin of ARGs in the environment. It also summarizes the information related to the sources, proliferation, dissemination, and management strategy for reducing the release of the ARB and ARGs in aquatic environments.
Origin and function of ARGs in the environment
When ARGs were found, they were assumed to have developed in antibiotics producing bacteria to safeguard against the impacts of antibiotics. The critical role of ARGs in environmental settings is to modulate responses produced from sub-Minimum Inhibitory Concentration (MIC) of antibiotics. Several ARGs may take part in modulatory functions in the biosynthesis of antibiotics [9]. It has been recommended that β-lactamases are enzymes engaged in the synthesis of peptidoglycan. It has been exhibited that sub MIC of antibiotics, around 200 folds below MIC values, can develop ARB. ARGs conferring resistance to tetracycline’s, β-lactams, and glycopeptides have been observed in very old Beringian permafrost. ARB has also been observed in a cave in New Mexico, the USA, confined for more than 4 million years. Based on the above studies, the environment is treated as a potential ARG reservoir from which disease-causing bacteria may employ prevention against antimicrobial agents applied against them by humans.
Sources of ARB and ARG in the aquatic environment
Freshwater and wastewater environment: The animal and human microbiome is a potential pool for ARGs. Bacteria crossing via the intestinal tract can assimilate antibiotic resistance and culminate in animal and human feces. The utilization of antibiotics can exert a selection pressure inside the gut, directing the propagation of antibiotic resistance in gut bacteria. Wastewater produced in homes, farms, hospitals, and other facilities comprises ARB and ARGs. A met genomics study conducted in Wastewater Treatment Plants (WWTPs) in sixty countries described the differences in ARGs occurrence and diversity between Asia/Africa/South, North America/Europe and Oceania. North America/Europe and Oceania cluster covered a narrow range of ARGs encoding macrolide resistance in great abundance. In contrast, Asia/Africa/South America cluster covered ARGs encoding resistance to phenols and sulfonamides. The study also reported that India, Vietnam, and Brazil have the most uneven distribution of ARG and indicated them as potential hotspots for the development of recent antibiotic resistance [10]. WWTPs endure chemical and microbial loads from diverse sources, exerting the selection pressure of antibiotic residues, heavy metals, and other chemical constituents and facilitating Horizontal Gene Transfer (HGT). Many studies have supported that WWTPs cannot wholly eliminate antibiotic residues, ARB, and ARGs. Thus, WWTP effluent release to environmental compartments, including soil, surface water, and groundwater, where environmental microbes can amalgamate with ARB and ARGs that develop antibiotic resistance.
Oceanic environment: The existence of ARB and ARGs in oceanic environments can have diverse mechanisms than wastewater and freshwater. Based on a previous study, merely 28% of the ARGs detected in oceanic environments were formerly studied [11]. Littoral overflow of ARB from terrestrial origins has been recognized as one of the mechanisms for the prevalence of antibiotic resistance in oceanic environments. Furthermore, marine aquaculture-related activities lead to the anthropogenic antibiotic runoff-related selection. Consequently, a few ARGs are initiated in the water environment and might have entered the anthropological environment. Briefly, critical origin of ARB and ARGs should be veterinary and human clinical settings where gut bacteria are subjected to elevated concentrations of antibiotic residues, land wastes, and WWTPs. However, current studies depicting worldwide antibiotic pollution in aquatic environments recommend the propagation of ARB in aquatic environments [12].
Distribution pathways of ARGs entering the environment
ARGs originated from a human source can flow into the environment via several different routes. They are liberated from hospitals as hospital wastewater. Also, ARGS are discharged into the WWTPs via the ingestion of antibiotics by the human. Antibiotics can finish up in sludge distributed on agricultural fields as fertilizer or discharged as runoff from WWTPs into the surface waters. Wastewater can also be treated in wetlands. Antibiotics are also utilized clinically or as growth regulators in poultry and livestock. Antibiotics and their determinants will proliferate through animal excreta and finish up in agricultural fields and groundwater or directly into the water environment in the case of antibiotics utilized in aquaculture. It is observed that ARB accompanies the same distribution routes wherever antibiotics are dispersed. It leads to environments where antibiotics, ARB, and ARGs are combined. These environments are regarded as resistance hotspots where ARGs imitate by producing new resistant microbial strains through HGT. There are several pathways through which humans may reach into contact with these resistant strains (Figure 1). They comprise ingestion of crops cultivated by polluted sludge used as fertilizer, potable water withdrawn from the polluted surface or groundwater, and cavorting in sea water associated with polluted surface water. Once resistant strains enter humans, they have the chance to proliferate their ARGs to the human microbiota [13].
Figure 1: A pathway of ARGs entering the environment.
Risk assessment
It is the qualitative or quantitative delineation and prediction of probable health hazards correlated with individuals or population’s subjection to hazards. It is generally achieved using the QMRA approach, which integrates the hazard identification, exposure, and dose-response assessment to evaluate the probable health hazards caused by ARB or ARGs. QMRA has been considered an appropriate method for quantitative estimation of the human health hazard caused by the consumption of ARB in the environmental compartments. The study evaluated that ARG transmission rates vary from 10-2 to 10-9 linked with bacterial counts and estimated the likelihood of ARG transfer to probable bacterial pathogens ensuing oral consumption. The earlier study also demonstrated that even 10 Escherichia coli particles are enough to affect an individual in a few cases. The aptness of the QMRA method in assessing the human health hazards caused by ARB and ARG numerically in aquatic environments is constrained due to the limited accessibility of exposure and dose-response information of ARB and ARGs for diverse settings (Table 1). QMRA comprises of a series of interrelated steps, which are described below in brief:
• Hazard identification.
• Exposure assessment.
• Dose-response assessment.
• Risk characterization.
| Hazard identification | The method of estimating whether or not contacts to a harmful physical biological, chemical, or biological agent. |
| Dose-response assessment | The method of identifying the link between the administered dose and the prevalence of harmful health consequence in exposed peoples. |
| Exposure assessment | The method of determining the frequency and intensity of human contacts to an agent presently existing in the environment. |
| Risk characterization | The method of measuring the occurrence of health consequence under the several circumstances of human contact. It is accomplished by combining the above three steps. |
TABLE 1 Generalized criteria for risk assessment
Hazard identification: It involves identifying those physical, chemical or biological agents that could probably cause a hazard to the people or organisms, identifying how hazard could happen. Antibiotics determinants could hasten the manifestation and development of ARB and ARGs in environmental settings [14]. The hazards linked with the environmental antibiotic resistome signify the transfer of environmental ARB and ARGs to humans. Antibiotics determinants in environmental compartments generate selection pressure on the environmental microbiota and thus create environmental pools of ARB and ARGs. However, the transmission of environmental ARGs to humans could be carried by pathogenic ARB or human dependent ARB. Antibiotics determinants that accompany the development of ARB hazards. The gene copy number of ARGs should be characterized as the principal hazard for assessing risk to exposed populations through water ingestion. Factors that are pondered necessary include the range of possible pathways involving the discharge of antibiotics and their residues, ARB, and ARGs into and augmenting in environmental settings including plants, birds, wildlife, soil, aquaculture, compost, wastewater lagoons, rivers, and sediments.
Exposure assessment: For a specific human health risk evaluation of ARB and ARGs, it would be significant to consider individual pathway settings (identification of crucial environmental compartments to human interaction) pertinent to the antibiotic resistance residues detected in the problem identification and hazard analysis phases. Compartments of possible apprehension include compost, lagoons, and rivers, soil environments acquiring bio solids or animal manure, and sediments receiving wastewaters. More conventional pathways of human acquaintances to pollutants that could include environmental ARB and pathogenic ARB are potable water, irrigation, and recreational waters affected by sewage, food, and exposure to farm animal manure. What are emergent as an essential research disparity is the in situ growth of ARB within bio-films and their related free-living protozoa that may safeguard and transfer ARB to and within potable water systems. The later pathway could be especially ambiguous for hospital potable water systems where a previously susceptible population is revealed. Besides, with the growing use and introduction to locally collected rainwater, the atmospheric outcome of ARB may seed household systems [15]. During this step, there is a need to enumerate the ARB or ARGs acquired by the population via consumption or contact through diverse methods, including drinking or recreational pursuits. It estimates the risk in best and worst-case settings based on the gene copy number of ARG in the exposure pathway. The lowest number of gene copies in the subjected medium was considered as a best-case scenario. In contrary, the highest gene copies number observed was employed to estimate the worst-case scenario.
Dose-response assessment: It is essential to select hazards for which doseresponse health information is described stochastically, as accessible for the reference enteric pathogens (C. jejuni, S. enterica, E. coli) to depict the risks precisely. It is a fundamental component of the characterization of risks. It was accomplished to estimate the correlation between the dosage of ARGs copies ingested by the acquainted population and the probability of infection. It may articulate the levels where the response measurement is nearly unaffected compared to the control estimation. Initially described the β-Poisson dose-response model to evaluate Escherichia coli contamination in this study. The probability of infection was thus estimated using the model presented in the equation given below:
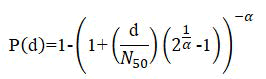
Where P (d)=Probability of infection, d=a dosage of ARG in exposure medium, N50=The median dose of infection and α=0.395, a dimensionless infectivity constant.
Risk characterization: It integrates the outcomes from hazard detection, hazard characterization, dose-response, and the exposure evaluation to produce an inclusive approximation of the risk. The approximation may be articulated in several risk measures, such as individual or population risk, or a measure of annual risk based on exposure to a particular hazard. It can also incorporate the critical scientific hypotheses employed in the risk assessment, sources of inconstancy and uncertainty, and a scientific assessment of risk management possibilities depending on the purpose of risk assessment. It further associates the hazard identification, exposure, and dose-response information to present a comparative or quantifiable estimate of risk, comprising of the sternness of the adverse outcome (consequences or impact) inclusive in the subjected population [16]. It may include estimating the number of individuals who undergone the health impacts over time or assesses signifying environmental damages. During this process, the understanding of the ARGs infection for the acquired population was accomplished by integrating outcomes of the hazard identification, exposure evaluation, and dose-response assessment that was executed independently for the different exposure scenario.
Challenges in tracing sources of ARGs in the environment
When ARB and ARGs are discharged into the environment, they spread into the aboriginal microbial populations via ecological and physiological interfaces fostering augmented genetic exchange. Furthermore, ARGs will be exposed to the dynamic environment over specific temporal and spatial magnitudes such as seasonal rainfall, river flow, and anthropogenic activities. All these phenomena will pose a series of problems in tracing the source of ARG in an environment.
Intervention of aboriginal ARG level: Antibiotic resistance is an inherent process preceding the widespread antibiotic usage in present medicine; hence, a specific type/level of ARGs is indigenous. It is essential to clarify interference from aboriginal genetic content to detect ARGs, particularly as contaminants from other sources. Clinically relevant ARGs have been observed in intrinsic environments. In natural soil, a highly different assemblage of genes imparting resistance to tetracycline, β-lactam, and glycopeptide antibiotics has been identified. In studies of the contaminated area across the geographical scale, the occurrence of an aboriginal Tet gene pool in the backdrop environment was detected, and the selection pressures sustaining these pools may work autonomously of the influence of different pollution sources, including lagoons and WWTPs. The study compared ARG levels in primeval upstream areas of the Cache-La-Poudre River and agricultural sites to deal with the background effect. Therefore, involving territorial control samples to differentiate aboriginal and introduced ARG in an environment is crucial for the source tracing of ARGs. Furthermore, primeval environments can be susceptible to human interference, as microbial populations and the resistance profile of the uncontaminated lake water can quickly shift under the relative acquaintance of treated wastewater [17]. Thus, locating the source of environmental ARGs involves a comprehensive inspection to detect a primeval natural environment.
Impact of spatial and temporal variation: In the majority of source-tracing studies of ARG, the sampling was performed within a particular area during a specific period to get a representative sample incorporating a pollution gradient. A broad investigation of spatiotemporal changes is mandatory for planning a successful sampling campaign to ensure that ARGs profiles in collected samples redirect the magnitude of anthropogenic hindrances in the environment. Landscape topographies including population density and distribution, sewage systems, animal husbandry, and pharmaceutical businesses can directly influence. Seasonal climate influence and human activities may employ a supplementary force in enhancing or reducing ARGs in a particular region. A substantial difference was recorded in the behavior of ARGs during the rainfall-runoff episodes in an urban inland stream, in which rainfall-mediated transmission of ARGs encompassed a substantial fraction of an entire downstream influx. Thus, special consideration should be given to understand the variation in ARGs profile between wet and dry seasons, especially in tropical regions. Furthermore, under or over-evaluation of human effect on ARGs pattern might occur without accounting other essential hydrogeological features. In the study on the Mississippi River, where very high ARGs levels are present in wastewater effluents, experimental evidence exhibited that these ARGs inputs had a negligible influence on the ARGs copy number. A one-dimensional model revealed that the main cause for the unanticipated outcome was more river flow rate than wastewater discharges. In addition to the changes in an environmental setting, the variation in biomass concentration along temporal and spatial scales might influence ARGs development.
ARG occurrence and perseverance determined by co-selective agents: The impact of the co-selective agents on the proliferation of ARGs growth has been documented in recent years. Frequent genetic co-occurrences and experimental evidence imply that the heavy metal exposure can infer resistance to a broad class of antibiotics [18]. Some well-distinguished mechanisms for AMR have been suggested for metal-induced co-selection, which primarily includes co-resistance, cross-resistance, and co-regulation. Moreover, biocides may mimic antibiotics in their action mechanisms and facilitate ARGs maintenance. These documented studies recommend that other contaminants signify a comprehensive and intractable selection pressure leading to ARGs propagation and distribution in the environment. It has been illustrated in tracing the source of ARGs effluence in WWTPs, and polluted farming areas, where ARGs proliferation was ominously associated with non-antibiotic agents. The study was performed to observe the influence of the Colorado Front Range flood on the riverine dissemination of ARGs; quantitative monitoring recommended selection pressures applied by heavy metals help reinstate of ARGs profile. It is further confirmed by the co-occurrence of heavy metal and ARGs in network assessment. Thus, co-selective agents might be an influential factor in the manifestation and perseverance of ARGs in the environment [19].
Inherited heterogeneity in environmental ARGs contamination: A broad picture of AMR in a specified environment is an aggregation of resistance genes designated as 'resistome', incorporating both inherent and developed ARGs. Extensive met genomics studies on resistive amongst several habitats have exhibited ARGs remarkable breadth and depth beyond clinical settings. Significantly, urban WWTPs and livestock farming often have an enormous ARGs diversity. A relatively high microbial population in these environments significantly amplifies the probability of transmitting and maintaining such distinct ARGs compared to other environments. ARGs contamination is an association between ecologically linked resistome, which single gene based methods can barely tackle (Table 2). Regardless of attempts by incorporating several ARGs in source tracing, the frequently inconsistent annotations centered on distinctive single marker resistance genes even tangled source detection [20].
| Challenges | Solutions |
|---|---|
| Autochthonous vs. allochthonous ARG | Identify background ARGs level in the pristine environment. |
| Geographical variability | Detail landscape survey. |
| Temporal variability | Record of human and climate activities. |
| Co-selective agents | Include non-antibiotic contamination. |
| Genetic diversity | Evaluate the overall ARGs profile. |
| Accurate source tracking | Source tracking of environmental ARGs using marker gene, and metagenome guided methods. |
TABLE 2 Challenges and solutions of accurate quantifiable source tracing of environmental ARGs contamination
Management strategy for lowering the discharge of ARG into the aquatic environment
The environmental contamination could be restricted by implementing solutions at several levels, from antibiotic usage to waste discharge comprising (Table 3). Thus, the solutions pointing to decreasing the environmental contamination by ARB and ARGs will be reflected at three stages:
• The abatement of antibiotic usage in plant, animal, and human health.
• Control and monitoring of wastes comprising ARB and ARGs
(agricultural, hospital, urban, industrial wastes).
• Managing the polluted environment (wildlife, soil, aquatic milieu).
| Environment | Activities or products monitored |
|---|---|
| WWTPs | Release of treated and untreated effluent (industrial and municipal sewage) to land or water bodies. Wastewater reuse. Land application of sludge/biosolids. Thermophilic anaerobic sludge digestion. Membrane separation and ozonation. |
| Agriculture | Land dispersal of sewage sludge and animal manure. Anaerobic digestate as biofertilizer. Bioaerosols from composting and animal farming. |
| Animal husbandry | Containment of animal wastes. Disposal of animal by-products, slurry, and manure. Digestion of livestock waste. Application of manure separation technologies. |
| River water quality | Impact of industrial and municipal sewage effluent. Impact of dispersed contamination from the farmyard, biosolids and manure. Storm water runoff. |
| Groundwater quality | Percolation of soil conditioner (manure and biosolids). Chemical treatment of crops. |
| Coastal and bathing waters | Control of sewage effluent and farmlands runoff on bathing and recreational water quality. Impact of aquaculture on the marine water quality. |
TABLE 3 Management options to prevent the proliferation and control of ARGs in the environment
It is currently flattering apparent that the environment enacts a vital function in the propagation and distribution of ARGs. A piece of emergent information recommends that ARGs are universal in environmental settings. Several studies have accomplished to relate greater concentrations of environmental ARGs to sites disturbed by anthropogenic behaviors. These enhanced levels are anticipated to ascend from the monotonous release of antibiotics, ARB, and ARGs into water environments through wastewater and runoff from animal livestock. These anthropocentric pollutants produce a potential hotspot for disseminating antimicrobial resistance and building up the environmental reservoir of ARB and ARGs. Contamination with elevated concentrations of antibiotics is of perceptible apprehension as such concentrations are liable for proliferation of resistant bacteria. In contrast, it is vague that how low concentrations of antibiotics influence the microbial population in multifaceted environments. Apprehending the function of the aquatic environment in disseminating ARGs is vital to efficiently reduce the emerging risk of antibiotic resistance. Ingestion of ARB and ARGs existing in aquatic environments can cause a significant human health hazard. Presently, it is difficult to quantifiably estimate human health risk using QMRA method due to the limited accessibility of required data on exposure and dose-response analysis for diverse settings. Moreover, unknown hotspots are playing an important role in the dissemination of antibiotic resistance in environmental compartments. However, characterizing ARB as a contamination marker will benefit human health risk evaluation. Various management alternatives across aquaculture, water and wastewater treatment, and pharmaceutical manufacturers could help in alleviating antibiotic resistance in the environmental compartments.
The authors thank the University Grant Commission (UGC), India for providing financial assistance (UGC-Ref. No.: 24625/(NET-DEC. 2013)) for this study.
The author declares that they have no known competing financial or personal interests.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Ranjan R. Health risk assessment framework and management strategy for the antibiotic resistance genes in the aquatic environment. AGBIR.2024;40(3):1116-1120.
Received: 30-May-2023, Manuscript No. AGBIR-23-100711; , Pre QC No. AGBIR-23-100711 (PQ); Editor assigned: 02-Jun-2023, Pre QC No. AGBIR-23-100711 (PQ); Reviewed: 16-Jun-2023, QC No. AGBIR-23-100711; Revised: 31-Jul-2023, Manuscript No. AGBIR-23-100711 (R); Published: 07-May-2024, DOI: 10.35248/0970-1907.24.40.1116-1120
