Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2024) Volume 40, Issue 6
Artemisia annua L. is an annual plant originary from China and mainly cultivated for its antimalarial properties. The present study aims to promote the efficient production of aerial biomass and viable seeds of the species. Artemisia annua plants were evaluated in a factorial block with three replications under two factors. This is water stress with three modalities (unstressed, stressed during vegetative stage, stressed during flowering stage) and organic fertilizer with also three levels (no fertilizer, compost, fertile soil). The results showed that unstressed plants fertilized with fertile soil significantly produced (p<0.0001) more above-ground biomass (31.91 ± 0.64 g) than unstressed plants fertilized with compost (20.90 ± 1.24 g) and unstressed and unfertilized plants (6.41 ± 0.52 g). In addition, transpiration by unstressed plants was greater in the presence of organic fertiliser, with average quantities of water loss of 14.12 ± 1.2 g per hour in the fertile soil and 11.27 ± 1.0 g per hour in the compost, compared with 8.95 ± 0.7 g per hour in the control. We also found that seeds of plants in fertile soil and stressed at the flowering stage had a significantly (p<0.0001) higher germination rate (92.74 ± 3.79%) than the unstressed and unfertilized control plants (54.69 ± 2.65%). The results obtained from this study could be used for Artemisia production and vulgarization.
Artemisia annua; Biomass; Fertilization; Abiotic stress
Artemisia annua L. is a member of the Asteraceae family. It is an aromatic herb occurring in cool temperate and subtropical regions [1]. This species is largely over large areas in countries such as China, Kenya, Tanzania and Vietnam [2]. It has been used in traditional medicine in China for thousands of years to treat malaria and fevers, due to its active ingredient, the artemisinin molecule. Malaria remains a global health problem, responsible for the deaths of several million people every year [3]. Africa remains the region with the highest malaria burden, with an estimated of 234 million cases of malaria and 593,000 associated deaths in 2021 [4]. In Burkina Faso, more than 3.5 million cases of malaria have been recorded, including 1,002 deaths [5].
The most effective treatment recommended by World Health Organization (WHO) since 2001 to fight against malaria is artemisinin-based combination therapy [6]. The artemisinin, which was discovered in 1972 is nowadays the main component of pharmaceutical drugs including Artefan, Artemether and Coartem. It is used in combination therapy Artemisinine based Combined Therapy (ACT) with other antimalarial drugs [7]. Several studies have also demonstrated the clinical efficiency of Artemisia annua tea in the treatment of malaria [8,9]. Despite its potential and high level of exploitation, production of the plant biomass and seeds is still unable to meet national demand. Large-scale production of this exotic plant is therefore necessary. Hence the importance of testing the acclimatization of this plant in West Africa. The general aim of this study was to identify an efficient method for producing the plant biomass and seeds. Specifically, the study aims to
• Determine the effect of each type of fertiliser on above-ground biomass and seed biomass production.
• To establish the impact of water stress on above-ground biomass and seed biomass production.
• To test the effect of fertilization and water stress on the germination quality of plant seed.
Study sites: location and climatic characteristics
A nursery was first set up in the experimental garden of the University Joseph KI-ZERBO in Ouagadougou located between the longitude 1°29 W and the latitude 12°22 N. Seedlings were then transferred to the experimental site in Ipelcé, located at 45 km away from Ouagadougou. The locality is located in the center-south region, more precisely in the province of Bazèga. The site is located in the 700 mm north and 1000 mm south isohyets, with geographical coordinates of 1°32 west longitude, 11°55 north latitude and an altitude of 318 metres. The province is part of the northern Sudanian sector [10].
Plant material
The plant material consists of certified hybrid seeds of Artemisia annua from the Apollo variety and the Mediplant variety acquired from the agroecological association "Beo neere" specialising in the production and marketing of Artemisia annua L. seeds. The choice of this hybrid species is related to the high artemisinin content of its seeds compared with other species.
Technical equipment
The technical equipment used for the trials consisted of: Germinators in tubs for germinating the seeds, a high-pressure sprayer for fine watering of the germinating seeds, a thermo-hygrometer for measuring the temperature and relative humidity of the air, a tape measure for measuring the height of the plants, one-liter plastic bags for transplanting the young plants and a watering can.
Conducting the trial
Setting up the nursery and caring for seedlings: The seedlings were sowed in germinators drilled at the bottom and one-third filled with substrate. During the nursery period, the plants were watered twice a day with a pressure pump until the 5-leaf stage.
After 08 weeks in the nursery, the plants were transplanted into bags to encourage their growth.
Organic fertilization: Two types of organic fertilizer were used to test good plant growth and development Compost (Co) and Fertile Soil (SF). Fertile Soil is a type of compost produced on a semi-industrial scale by the agricultural company AROMH/SOLFERTIL. The organic fertilizers were applied after transplanting the seedlings into 5-litre plots.
The Quantity of Organic Fertilizer applied per pot (QFO/pot) was determined using the following formula:
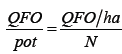
Where QFO/ha is the quantity of fertiliser per hectare and N the total number of pots per hectare.
To obtain optimum results, the Food and Agriculture Organization (FAO) recommends. The dose of organic fertilizer used in our trial was 500 kg/ha following the recommendation of FAO to apply a handful of compost per planting bed (400 to 600 kg per hectare) to obtain optimum results [11]. The quantity of organic fertilizer applied per pot in the trial was 25 g with QFO/ha=500,000 g and N=20,000.
Soil and fertilizer composition: The results of the chemical analyses of the soil show that the substrate in the study pots has a soil pH very close to neutral (Table 1). The soil and the organic fertilizers used are relatively rich in Organic Matter (OM), Carbon (C), Nitrogen (N), available Potassium (K) and assimilable Phosphorus (P). However, there is more organic matter in compost. Fertile soil has the highest levels of available Nitrogen (N) and Phosphorus (P).
| Total OM (%) | C total (%) | Total N (%) | C/N | Assimilable P (%) | Assimilable K (%) | pH water | |
|---|---|---|---|---|---|---|---|
| Soil | 2.016 | 1.169 | 0.063 | 19 | 0.1006 | 0.9577 | 6.06 |
| Fertile soil | 50 | 30 | 3 | 10 | 4 | 2 | 7 |
| Compost | 53.22 | 30.87 | 1.81 | 17 | 2.57 | 2.56 | 7.71 |
Note: OM=Organic matter; C=Carbon; N=Nitrogen; P=Phosphorus; K=Potassium.
Table 1: Chemical characteristics of soil and fertilisers.
Application of water stress: Three types of water treatment were applied including unstressed plants (Controls), plants stressed at the vegetative stage and plants stressed at the flowering stage.
Control plants were watered twice a day at field capacity morning-evening throughout the trial. Plants stressed at the vegetative stage were watered from transplanting into pots until the stage when leaf biomass was well developed (i.e., 98 days after sowing) before the application of water stress, which consisted of stopping watering the plants. Plants stressed at the flowering stage were watered normally from transplanting until the appearance of flower buds (117 days after sowing), at which point watering was stopped. Water stress was applied for four days at both stages, as the plants quickly reached the point of permanent wilting after four days without watering.
After the stress was stopped at these different stages, the plants were watered again in the morning and evening until the seeds reached maturity.
Experimental set-up: The experimental set-up used was a factorial block with two factors, namely water stress and fertilisers (Figure 1). There was a total of 9 treatments per block, three levels of stress (i.e., unstressed control plants, plants stressed at the vegetative stage and plants stressed at the flowering stage) and three levels of fertiliser (i.e. unfertilised control plants, plants fertilised with fertile soil and plants fertilised with compost). Each treatment has three pots as experimental unit.

Figure 1: Experimental set-up in a factorial block,  Te: Control; Co: Compost; SF: Fertile Soil; St Vg: Stressed vegetative stage; St
Fl: Stressed flowering stage; N St: not stressed.
Te: Control; Co: Compost; SF: Fertile Soil; St Vg: Stressed vegetative stage; St
Fl: Stressed flowering stage; N St: not stressed.
Seed viability test: After seeds harvesting, a germination test was carried out to ensure the quality of the seeds produced (germination capacity). At this stage, the seeds from the different treatments germinated. These are seeds from unstressed plants (controls) and seeds from stressed plants at the vegetative and flowering stages, fertilised with compost or fertile soil.
Before the germination test, 0.1 g of seed was weighed and mixed with three spoonfuls of sieved sand. Once the mixture was homogeneous, in-line sowing was carried out in traditional germinators (cut-out cans). To obtain more accurate estimates, we repeated the tests with different samples. The system used to carry out the germination test was a randomised complete block trial.
Data collection
Environmental parameters: As Artemisia annua is not a plant of Sahelian origin, the effect of temperature and relative air humidity is decisive for its adaptation.
Air temperature and relative humidity: The air temperature and relative humidity were recorded daily during the growth and development phases of the plants at 07:00, 14:00 and 18:00 respectively from July to October 2022. The instrument used was a THER-D31-001 thermo-hygrometer.
Growth parameters
Plant height: The height of plants (cm), whether unstressed and fertilised (either with fertile soil or compost) or not (control plants), was measured every week from the first to the fifth week. This was done after planting using a measuring tape.
Total dry biomass: Dry biomass (g) was determined after stripping the plants. This involved immersing the plant's root system in a container containing water for a few moments so that the soil separated easily from the root system [12]. Using secateurs, the roots were cut at the collar. The total biomass of the plants in the pots was obtained after drying for a week in the shade. The biomass of the seeds was obtained after a light rubbing, by hand, applied to the fruits located on the branches, which had previously been dried under shade. Above-ground and root biomass were respectively obtained by sectioning the plant from the collar and stripping, followed by washing the root part with clear water to separate the roots from the growing medium. Aboveground and root biomasses obtained were dried under shade (to avoid destroying their active ingredients) for a fortnight.
Physiological parameters
Sweating: The transpiration of unstressed plants fertilised (either with fertile soil or compost) or not (control plants) was measured every hour between 7 am and 6 pm, based on variations in the mass of the pots measured at regular time periods. The pots, covered with plastic bags to prevent evaporation, were regularly weighed. Transpiration was determined gravimetrically on 98eme Days After Sowing (DAS). Pot mass losses were determined by the difference between the mass of a pot at time T1 and its mass at time T2. The time between T1 and T2 was one hour. The pots were weighed using a Sartorius electronic balance with a precision of 0.001 g.
Germination test: To assess germination capacity, three parameters were taken into account. These are:
Germination rate: To obtain the germination rate, the Artemisia house has found that in 0.1 g of seeds produced from certified seeds, a maximum of 30 seeds are able to germinate or are fertile. In the present trial, for 0.1 g of seed germinated, we obtained 140 seeds that germinated (fertile) for the greatest value. On this basis, we considered 150 seeds as the total number of germinated seeds. The germination rate was calculated using the formula:

Germination speed: Where Gi:number of seeds germinated on the day and Di = number of days after sowing.
Germination time: This is the time interval taken for the first germination.
The results of the germination test are expressed in terms of germination rate, germination speed and germination time.
Statistical analysis
Data entry and the construction of curves and histograms were performed using Excel (version 2016). An analysis of variance using the Newman-Keuls test with a threshold of 5% and a comparison of the means of the different treatments were carried out using XLSTAT 2016 software.
Environmental parameters
Air temperature and relative humidity:The daily averages of air temperature and relative humidity recorded during the experiment. The average temperature during the trial was 33.73 ± 2.14°C, with a minimum of 25.13°C and a maximum of 42.33°C. The average relative humidity was 58.88 ± 12.44%, with extreme values of 33.30% and 84.47% (Figure 2).

Figure 2: Air temperature and relative humidity,  .
.
Growth parameters
Height of plants: Figure 3 shows plant height curves for each treatment as a function of week. During the first week, plant heights were broadly similar, but the control had a significantly higher value (p<0.0001) (6.64 ± 0.87 cm) than the compost (6.36 ± 0.03 cm) and Fertile Soil (6.44 ± 0.09 cm). However, from the second week onwards, the plants fertilized with compost and fertile soil recorded the highest values (p<0.0001) (Table 2) up to the fifth week (i.e., 105eme days after sowing) with (23.39 ± 0.43 cm) and (22.22 ± 0.15 cm) respectively.
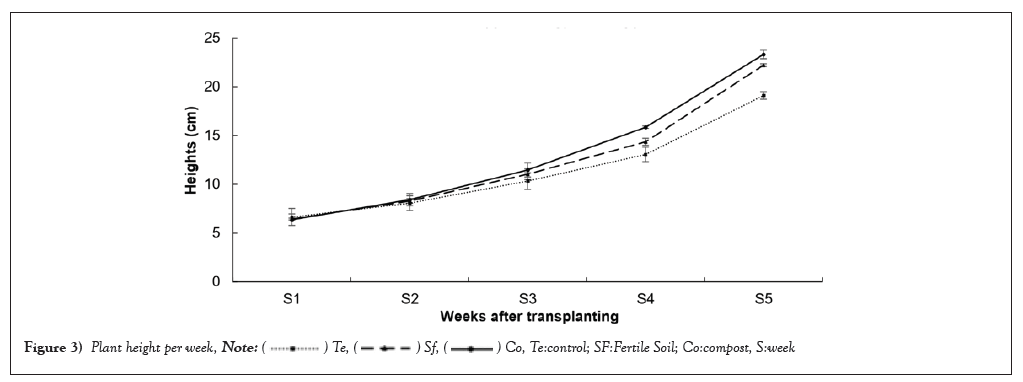
Figure 3: Plant height per week,  Te:control; SF:Fertile Soil; Co:compost, S:week.
Te:control; SF:Fertile Soil; Co:compost, S:week.
| Fertiliser | Average height at 105eme days after sowing |
|---|---|
| SF | 22.22 ± 01.15b |
| Co | 23.32 ± 0.43a |
| Te | 19.11 ± 0.008c |
| Pr>F | 0.0001*** |
Note: SF=Fertile soil; Te=control; Co=Compost; Values followed by the same letter in the same column are not significantly different at the 5% threshold; Pr: probability; ***: highly significant; a,b,c = significantly different at the 5% threshold.
Table 2: Average height at 105ème JAS as a function of fertiliser type.
Total dry biomass: The results of the analysis of variance (Table 3) show a significant difference between the different treatments. Unstressed plants fertilized with fertile soil or compost obtained the highest values of above-ground dry biomass (31.91 ± 0.64 g; 20.90 ± 1.25 g), root biomass (13.706 ± 1.38 g; 7.200 ± 1.01 g) and seed biomass (12.927 ± 0.27 g; 9.470 ± 0.88 g), respectively. On the other hand, whatever the fertiliser used, plants stressed at the vegetative or flowering stage, as well as the controls, recorded the lowest above-ground biomass values.
| Stress stage | Fertiliser | Seed biomass (g) | Above-ground biomass (g) | Root biomass (g) |
|---|---|---|---|---|
| No stress | SF | 12.927 ± 0.27a | 31.917 ± 0.64a | 13.706 ± 1.38a |
| No stress | Co | 9.470 ± 0.88b | 20.907 ± 1.24b | 7.200 ± 1.01b |
| No stress | Te | 3.290 ± 0.22d | 6.417 ± 0.52d | 1.765 ± 0.67e |
| Vegetative | SF | 7.059 ± 0.48c | 14.025 ± 1.7c | 4.100 ± 0.69cd |
| Vegetative | Co | 5.934 ± 0.97c | 12.591 ± 0.7c | 4.197 ± 0.54cd |
| Vegetative | Te | 3.426 ± 0.51d | 6,\.870 ± 1.42d | 1.893 ± 0.17e |
| Flowering | SF | 6.622 ± 0.68c | 13.185 ± 1.16c | 5.657 ± 1.67bc |
| Flowering | Co | 5.946 ± 0.76c | 12.079 ± 1.04c | 4.163 ± 0.79cd |
| Flowering | Te | 3.493 ± 0.99d | 8.033 ± 1.57d | 3.807 ± 1.2d |
| Pr>F | 0.0001*** | 0.0001*** | 0.0001*** |
Note: SF: Fertile Soil; Te: control; Co: compost. Flowering: stressed at flowering stage, Vegetative: stressed at vegetative stage. Values followed by the same letter in the same column are not significantly different at the 5% threshold; Pr: probability; ***: highly significant; a,b,c,d = significantly different at the 5% threshold.
Table 3: Components of dry biomass production in pots.
Physiological parameters
Sweating: Figure 4 shows the evolution of hourly transpiration during the day. In general, all the plants fertilized with fertile soil recorded the highest average transpiration value of 26 g/h, between 12 and 1pm. For plants fertilized with compost, an intermediate average value of 20.5 g/h was observed. The lowest average transpiration value (17.5 g/h) was obtained by the unfertilized plants (control). During all the hours of measurement, the transpiration of the fertilized plants was significantly higher than that of the control plants (Table 4).
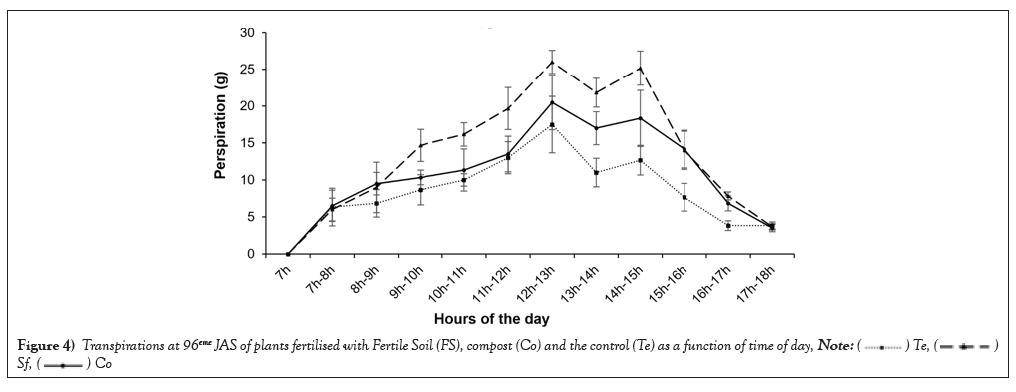
Figure 4: Transpirations at 96eme JAS of plants fertilised with Fertile Soil (FS), compost (Co) and the control (Te) as a function of time of day, 
 .
.
| Fertilisers | Average perspiration grams (g) in |
|---|---|
| SF | 14.12 ± 1.2a |
| Co | 11.27 ± 1b |
| Te | 8.95 ± 0.7c |
| Pr > F | 0.0001*** |
Note: SF=Fertile Soil; Te=control; Co=compost; g=gram. Values followed by the same letter in the same column are not significantly different at the 5% threshold; Pr: probability; ***: very highly significant; a,b,c = significantly different at the 5% threshold.
Table 4: Average transpiration at 96eme JAS as a function of fertiliser type.
Plants generally transpire between 12 noon and 1 pm. and 2 pm. and 3 pm., with the midday low observed between 1 pm. and 2 pm. This is the time of day when it is warmer and plants transpire less.
Germination test
Germination rate: Seeds from plants fertilized and stressed at the flowering stage had the highest germination rate. Plants fertilized with fertile soil and compost and stressed during the flowering stage had germination rates of (92.74 ± 3.79%) and (68.22 ± 1.53%) respectively. The lowest germination rate was observed for seeds from unstressed control plants, at 54.67 ± 2.14%. Plants stressed at flowering and fertilized with fertile soil and compost had the highest germination rates, at 19.78 ± 0.68 gr/dr and 13.94 ± 0.45 gr/dr respectively. The control, which was not subjected to stress, recorded the lowest germination rate (9.78 ± 0.53 gr/dr) (Figure 5).
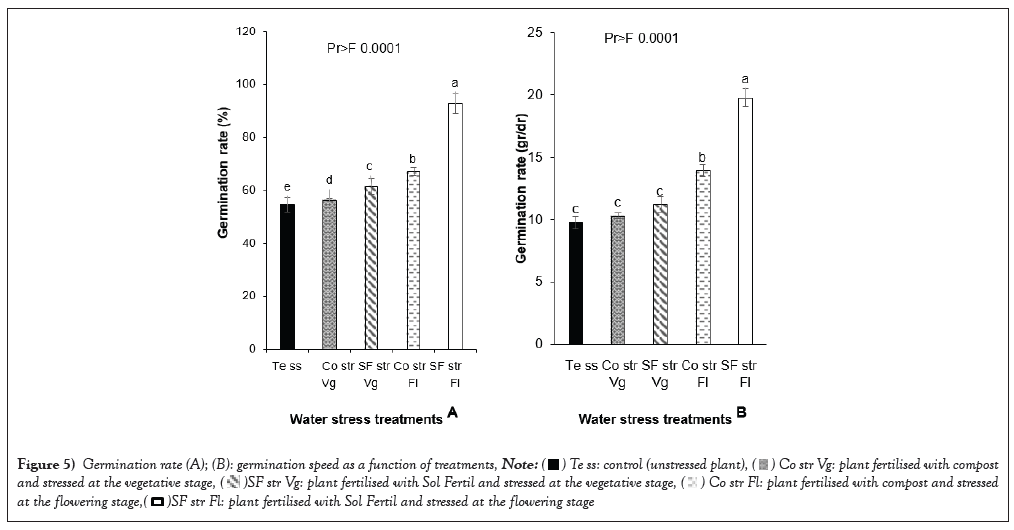
Figure 5: Germination rate (A); (B): germination speed as a function of treatments,  plant fertilised with compost
and stressed at the vegetative stage,
plant fertilised with compost
and stressed at the vegetative stage, 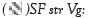 plant fertilised with Sol Fertil and stressed at the vegetative stage,
plant fertilised with Sol Fertil and stressed at the vegetative stage, 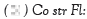 plant fertilised with compost and stressed
at the flowering stage,
plant fertilised with compost and stressed
at the flowering stage, 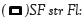 plant fertilised with Sol Fertil and stressed at the flowering stage.
plant fertilised with Sol Fertil and stressed at the flowering stage.
Germination time: The analysis of variance on the germination time of the germinated seeds (Table 5) showed a significant difference (p<0.0001) between the different treatments. Indeed, it was the plants stressed at the vegetative stage and at the flowering stage, and all fertilized with fertile soil that obtained a relatively low germination time, with 78.67 ± 1.53 h and 78.67 ± 0.58 h respectively.
| Treatments | Duration (h) |
|---|---|
| Co str Fl | 80 ± 1b |
| SF str Fl | 78.67 ± 0.58b |
| Co str Vg | 94.67 ± 1.53a |
| Te ss | 95.67 ± 2.08a |
| SF str Vg | 78.67 ± 1.53b |
| Pr>F | 0.0001*** |
| Significant | Yes |
Note: Tess: control (unstressed plant); Co str Vg: plant fertilised with compost and stressed at the vegetative stage; SF str Vg: plant fertilised with Fertile Soil and stressed at the vegetative stage; Co str Fl: plant fertilised with compost and stressed at the flowering stage; SF str Fl: plant fertilised with Fertile Soil and stressed at the flowering stage; ***: Very highly significant. Values followed by the same letter in the same column are not significantly different at the 5% threshold; Pr: probability; a,b = significantly different at the 5% threshold.
Table 5: Germination time according to type of treatment.
The results of the analysis of the composition of the various fertilizers show that they are relatively rich in organic matter. However, the mineral content of the soil at the experimental site shows a high C/N ratio of 19. This means that there is not enough nitrogen or that carbon mineralization is slow. Only a small amount of nitrogen is returned to the soil. Fertial and International Food Code (IFC) stated in 2010 that plants need macro elements such as nitrogen to ensure their development. In this study, the soil at the site had a low percentage of nitrogen, which may have contributed to reduce height growth and total biomass production. Root dry biomass production was higher in unstressed plants fertilized with Fertile Soil (13.70 ± 1.38 g) or compost (7.2 ± 1.01 g) compared with the unfertilized control (1.76 ± 0.67) g. This could be due to the lack of nutrients in the soil and the restriction on expansion imposed by the pot. Indeed, Ganse et al., [13] stated that the development of Artemisia annua L. is sensitive to environmental variables including the mineral content of the substrate. Soil amendment with organic fertilisers therefore enable root biomass production, enabling the root systems to extract more nutrients and water to ensure plant growth and development. This is undoubtedly what enabled fertilized plants to grow significantly taller than plants growing on unfertilized control soils. From the very first week, the plants in the control soils recorded the highest heights. This could be explained by the fact that, at that time, the fertilized plants had not yet incorporated the various nutrients present in each fertilizer, or that mineralization had been slow and would not have enabled the plant to obtain the mineral elements immediately. On the other hand, by the fifth week, plants fertilized with compost and fertile soil had gained the upper hand in terms of height, with 23.32 ± 0.43 cm and 22.22 ± 0.15 cm respectively. By using carbon and nitrogen, the micro-organisms break down the organic matter and release the other nutrients it contains. These nutrients then become available to plants. It is through this process that organic fertilization nourishes plants [14]. Given that the compost contained more organic matter, this would have contributed to the plants fertilized by it being taller than the others. Measurement of plant leaf transpiration revealed that plants transpired significantly (P<0.0001) more during hot periods of the day and reduced their transpiration by partial closure of the stomata corresponding to the “midday depression” during the hottest periods of the day (between 1 pm and 2 pm). Kihindo and Darrigan et al., [12,15] also reported a “midday depression” observed in ground ivy and cowpea respectively when transpiration thresholds are reached on a hot and/or dry day, enabling the plant to avoid water stress. Transpiration was highest in plants fertilized with fertile soil, followed by plants fertilized with compost, and lowest in unfertilized control plants. This may be due to the fact that the fertile soil and compost fertilizers produced more root biomass thanks to their physico-chemical properties, which improve water retention for rhizogenesis. The high production of root biomass induced by fertile soil and compost would have allowed to a high uptake of water and mineral elements by the plants they fertilized, thereby stimulating a high production of aerial biomass. Substrates with good water retention capacity that can be easily used by growing plants are capable of producing 150 kg of dry leaves per hectare on dry land [16]. The high production of above-ground biomass induced by the fertile soil or compost may have enabled the plants to transpire more. This high transpiration would certainly have led to a high gain in CO2, and therefore to good photosynthesis. According to several studies Farquhar et al., Bousba et al., [17,18] although the opening of the stomata allows water loss, it is the pathway for assimilating CO2 from the ambient air, which is necessary for photosynthesis. In this study, this is what led to high growth rates in plants fertilized with fertile soil or compost from the fifth week onwards. The germination test carried out on the seeds production showed that there was a highly significant difference (p< 0.0001) between the different treatments. It was the seeds obtained under stress conditions that produced the higher values. In fact, for the germination rate, it was the plants fertilized with fertile soil and stressed at the flowering stage that recorded a good rate. This could be due to the fact that water stress developed a species survival mechanism in these plants. According to Nguinambaye et al., [19] the plant gives priority to the seeds during water deficit by accumulating carbon and protein substrates in the seeds during stress. Everything is done as if the plant wanted to preserve the vital organs to safeguard the species. This is consistent with the results obtained in this study. Plants under water stress recorded the highest germination rates. The highest germination rate was also obtained with seeds from plants fertilized with fertile soil and stressed at the flowering stage. According to Sounon et al., [16] seed germination rate and speed evolve simultaneously. Dow El Madina et al., [20] also reported abortion or arrested development of flower buds in the event of high temperatures at the start of the reproductive phase. However, these results differ from those obtained in this study, where plants stressed during periods of high heat (maximum mean temperature of 42.33°C) produced viable seeds with the best germination rates and the shortest germination times at the vegetative stage (78.67 ± 1.53 h) than at the flowering stage (78.67 ± 0.58 h). Thus, seed viability and germination capacity are higher the more extreme the environmental conditions [21].
Artemisia annua L. is an aromatic plant widely distributed in cool temperate and subtropical regions of the world. This study shows that it is possible to produce dry biomass from this variety in the northern Sudanian zone. Fertilization improves biomass production. In fact, plants growing in soil (in pots), unstressed and fertilized with fertile soil produced more above-ground biomass, a part of the plant that is very useful for combating malaria. As for the germinative quality of the seeds produced, water stress significantly increased the germinative capacity of the seeds produced. The results of this study could be supplemented and reinforced through analysis of the biochemical composition of the biomass and trials in other phytogeographical areas.
[Crossref] [Google Scholar].
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Kihindo AP, Sawadogo N, Dondasse E, et al. Organic fertilisers and water stress effects on seed viability and plant growth of Artemisia annua L. in Burkina Faso. AGBIR.2024;40(6):772-778.
Received: 27-Nov-2023, Manuscript No. AGBIR-23-121229; , Pre QC No. AGBIR-23-121229 (PQ); Editor assigned: 29-Nov-2023, Pre QC No. AGBIR-23-121229 (PQ); Reviewed: 12-Dec-2023, QC No. AGBIR-23-121229; Revised: 19-Dec-2023, Manuscript No. AGBIR-23-121229 (R); Published: 27-Dec-2024, DOI: 10.35248/0970-1907.23.40.1351-1357
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
