Agricultural and Biological Research
RNI # 24/103/2012-R1
Research - (2023) Volume 39, Issue 6
The majority of instances of Tuberculosis (TB) occur in underdeveloped nations, and it is one of the leading causes of morbidity and mortality worldwide. In underdeveloped nations, it is still unclear how important pulmonary and extrapulmonary tuberculosis are compared to other types of the disease. The disease Tuberculosis (TB) is very contagious. Pulmonary tuberculosis is the name given to a disease that typically affects the upper respiratory system of the body. The most typical form of the disease is pulmonary TB. Mycobacterium tuberculosis, a slow-growing Acid-Fast Bacillus (AFB). The respiratory system is the route through which the Mycobacterium is spread. Extra-Pulmonary TB (EPTB) is the name given to a condition that can also lead to tuberculosis in an organ of the body. Identification and phenotypic characterization of clinically significant mycobacterium isolates from pulmonary and extra-pulmonary was performed along CBNAAT diagnosis. A total of 317 Pulmonary and Extrapulmonary specimens were received at IRL, Bangalore were used for this study. Smears were prepared for all pulmonary samples and smears were subjected to acid fast bacillus staining technique. Smear microscopy testing revealed 206 samples to be positive. Liquid culture media MGIT-960 was used to inoculate the 206 Mycobacterium samples that had positive smear results. Additionally, the Cartridge Based Nucleic Acid Amplification Test (CB-NAAT) revealed MTB in 91 Rif resistant, 170 Rif sensitive, 48 samples where MTB was undetectable and 5 samples where Rif intermediate was visible.
Mycobacterium tuberculosis; Phenotypic characterization; CBNAAT; Ziehl-Neelsen staining; Pulmonary tuberculosis
Tuberculosis (TB) is among the leading health problems, especially in low-income countries, and is considered as the first cause of death from an infectious disease and ninth cause of death worldwide. The emergence of Multi Drug-Resistant (MDR) form of this disease makes the treatment difficult even in developed countries [1]. Inappropriate use of antibiotics has led to the development of MDR-TB and Extensively Drug-Resistant Tuberculosis (XDR-TB). MDR-TB is defined as Mycobacterium tuberculosis strains that are resistant to the main first-line drugs (isoniazid and rifampin). XDR-TB strains are characterized by resistance to at least one of the three injectable aminoglycosides (kanamycin, amikacin, capreomycin) and Fluoroquinolones (FQs), as well as isoniazid and rifampin. The development of such resistant strains is a serious threat to the global control of tuberculosis [2].
One of the major health issues, particularly in low-income nations, is Tuberculosis (TB), which ranks first among infectious diseases, as well as ninth overall in terms of mortality rates. Even in affluent nations, the disease is difficult to treat due to the advent of Multi Drug-Resistant (MDR) forms [3,4]. The emergence of MDR-TB and Extensively Drug-Resistant Tuberculosis (XDR-TB) is a result of improper antibiotic use. Mycobacterium tuberculosis strains that are resistant to the two primary first-line medications (isoniazid and rifampin) are referred to as MDR-TB. The three injectable aminoglycosides (kanamycin, amikacin, and capreomycin) and Fluoroquinolones (FQs), as well as isoniazid and rifampin, are known to be resistant to at least one of the XDR-TB strains. The global control of such diseases is seriously threatened by the emergence of such resistant strains of tuberculosis [5,6].
The Quinolone Resistance Determining Region (QRDR) in the gyrA and gyrB genes of M. tuberculosis is mutated, with codons 90, 91, and 94 of the gyrA gene being the most often mutated. FQ resistance has been demonstrated to occasionally be linked to gyrB gene alterations [7]. The global prevalence of MDR-TB/Rifampin-Resistant TB (RR-TB) with resistance to any of the FQs ofloxacin, levofloxacin, and moxifloxacin reached 20% in 2017, according to WHO statistics. M. tuberculosis drug resistance is a critical barrier to effective TB therapy and control. Therefore, to stop the spread of the disease and to immediately begin effective drug therapy, prompt diagnosis of Drug-Resistant TB (DR-TB), especially FQs, is essential [8].
The Xpert MTB/RIF Assay (CB-NAAT), the LINE Probe Assay (LPA), and the Loop-Mediated Isothermal Amplification (LAMP) are among the Nucleic Acid Amplification Tests (NAAT) that are currently available and are recommended by the WHO for in vitro diagnosis of TB. The GeneXpert® system powered by Cepheid Innovations, for the CB-NAAT (Cartridge based), is an automated, semi-quantitative, hemi-nested, real-time PCR used for the concurrent detection of the MTB complex and its Rifampicin (RIF) resistance pattern associated with the mutation in the rpoB gene, in clinical samples with a turnaround time of 2 hours [9,10]. The goal of the current study was to characterise MTB phenotypically and diagnose it using CBNAAT using pulmonary and extrapulmonary samples obtained from different areas in Karnataka.
Sample collection
A total of 317 clinical specimens were received from various districts in Karnataka during the period of January 2022 to October 2022 at IRL, Bangalore were used for this study. 240 pulmonary and 77 extra pulmonary specimens for drug susceptibility testing, including treatment patients and presumptive patients were processed using standard methods. Pulmonary like sputum and extrapulmonary specimen like gastric aspirates, pleural fluid, bronchial lavage/ wash, pus, tissue, biopsy, ascetic fluid, lymph node, cervical fluid, endometrium, peritoneal fluids, pericardial fluid, synovial fluid and Cerebrospinal Fluid (CSF) will be included during the study period. Specimens will be centrifuged at 3000 g for 20 mins at 4°C using a refrigerated centrifuge and decontaminated by NALC-NaOH method. Same procedure was used for tissue samples after homogenization by cutting into small pieces and grinding with tissue grinder. CSF can be directly taken for further testing whereas it requires decontamination in case of turbid (visual appearance) CSF sample. Samples were processed in STDC-IRL, Bangalore, Karnataka, India. This study was conducted after the approval of the Institutional Ethical committee with ethical clearance certificate number 008/12/2021/IEC/SMCH.
Phenotypical identification of mycobacterium tuberculosis
Smear preparation: After performing Ziehl-Neelsen staining at the sample collecting sites, direct microscopic examination was employed for bacteriological analysis. In the refrigerators of the health institutes, a percentage of the positive samples were maintained at temperatures between 10 and 20°C. An expert pathologist took the utmost caution and safety when collecting the fine needle aspirations (FNAs) from patients suspected of having Extra-Pulmonary Tuberculosis (EPTB). In order to confirm its positive, the Ziehl-Neelsen smear method was used after that. For this study's purposes, a suction of approximately 1 ml samples was taken from positive patients and maintained in sterile, firmly closed capillary tubes with an equivalent quantity of phosphate buffer saline solution at pH 7.2 and kept in the same refrigerator. Sputum and FNA samples were then delivered via a cold chain at 4°C to TB, where they were stored in a deep freezer at -80°C prior to culture [11,12].
Primary inoculation to MGIT-960 systems
When specimens are being processed, the antibiotic supplements PANTA for the MGIT tubes were given. Each vial of PANTA was contained lyophilized mixture of the antimicrobials (Polymyxin B, Amphotericin B, Nalidixic acid, Trimethoprim and Azlocillin). MGIT PANTA was reconstituted with 15 ml growth supplement. MGIT growth supplement included OADC with POES (Oleic acid, Bovine Albumin, Dextrose and Catalase with Polyoxyethylene stearate).
MGIT tube was labelled and 0.8 ml of growth supplement mixture was added (PANTA and OADC with POES) using a micropipette with sterile barrier tip. 0.5 ml of well mixed sample was added. Tightly recapped the tube and gently inverted several times. The tubes were loaded to MGIT machine by scanning the barcode available on the MGIT tube.
CBNAAT diagnosis
The CBNAAT test uses Polymerase Chain Reaction (PCR) to identify TB and Rifampicin resistance. The CBNAAT device is an automated system for processing, amplifying, and detecting samples that is disposable, single-use, and self-contained. To liquefy and inactivate the bacteria in the sample, the sample reagent will be added in a 2:1 ratio to the sample before 2 ml of the sample is sampled into the cartridge and placed into the assay procedure equipment. The following steps are all automated. The following result patterns are used to group the test results: No-MTB found; MTB detected-Rifampicin resistance identified; MTB detected-No-Rifampicin Resistance found; MTB detected-Rifampicin Resistance Detected; MTB detected-Rifampicin Resistance Indeterminate; and an Invalid Result [13-15].
The development of methods to increase the effectiveness and sensitivity of smear microscopy is a top priority for global TB control [16]. In the current investigation, 317 specimens from patients with various presentations in total were examined. All samples were prepared for an acid-fast Bacilli direct smear. For all of the smears, the Ziehl-Neelsen staining method was used. A smear microscopy study on 206 of them revealed a positive result. 34 samples returned a negative result, and for the remaining 77 samples, no slide was created (Figure 1). The ubiquitous yet insensitive technique of sputum smear microscopy is still used to identify cases of pulmonary tuberculosis. The diagnosis of extrapulmonary instances, which is still a difficult problem for clinicians, is also not permitted. Even while extrapulmonary TB accounts for a sizable part of TB patients in some countries, it is typically underestimated due to diagnostic challenges. Although it lacks sensitivity, sputum smear microscopy is nevertheless often used to detect pulmonary TB patients [17]. Additionally, it does not permit the diagnosis of extrapulmonary instances, which is still a difficult problem for physicians. Despite accounting for a sizable number of TB patients in some countries, extrapulmonary TB is typically underestimated due to diagnostic challenges [18].
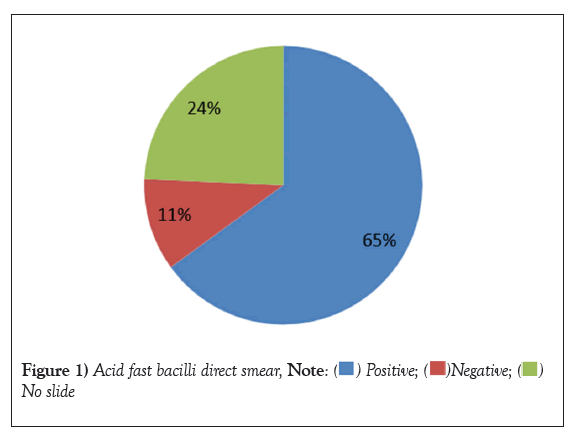
Figure 1: Acid fast bacilli direct smear,  No slide.
No slide.
Only 84% of the 100 lung samples with positive smear results demonstrated growth on liquid culture media MGIT-960; this study's low isolation rates can be attributed to sample cleaning with 4% NaOH or prior anti-tubercular therapy [19]. In the current investigation, 206 mycobacterium samples with positive smear results were grown on liquid culture media MGIT–960. Fluorescence, non-homogenous turbidity and small grains were observed in an inoculated MGIT tube for culture positive sample.
Numerous studies have been conducted on the best ways to diagnose and treat pulmonary tuberculosis. However, there is still much to be learned about extra pulmonary TB diagnosis. In order to investigate the effect of CB NAAT in detecting tubercular lymphadenopathy and rifampicin resistance in tubercular peripheral lymphadenopathy, research of 57 cases was done [19,20]. 96% (29 out of 30 patients) of the cases with tubercular peripheral lymphadenopathy in the FNA sample were positive for CB-NAAT [6]. According to a study by Claudia M. Denkinger et al., CB-NAAT has a sensitivity of 83.1% and a specificity of 93.6% when compared to TBLN culture [16]. In present study, CB-NAAT also detected MTB in 91 Rif resistance, 170 Rif sensitive, 48 samples MTB were not detected and 5 samples were showed Rif intermediate (Table 1).
| CBNAAT | ||
|---|---|---|
| Not detected | MTB not Detected | 48 |
| Rif resistance | MTB detected-Rif resistance | 91 |
| Rif sensitive | MTB Detected-Rif sensitive | 170 |
| Indeterminate | MTB Detected-Rif indeterminate | 5 |
| Error | 1 | |
| Invalid | 2 | |
| Total | 317 | |
Table 1: CBNAAT diagnosis of MTB from pulmonary and extra pulmonary samples.
In the current investigation, CBNAAT diagnosis was combined with the identification and phenotypic characterization of clinically significant mycobacterium isolates from the pulmonary and extra-pulmonary regions. 317 samples from patients with a range of presentations were examined in total. For samples, a direct smear with Acid Fast Bacilli was prepared. All of the smears were stained using the Ziehl-Neelsen method. Smear microscopy testing revealed positive results for 206 samples. The 206 Mycobacterium samples with positive smear results were grown on liquid culture media MGIT-960. Fluorescence, non-homogenous turbidity and small grains were observed in an inoculated MGIT tube for culture positive sample. MTB was also found using the cartridge-based nucleic acid amplification test (CB-NAAT) in 91 Rif resistant, 170 Rif sensitive, 48 samples where MTB was not found and 5 samples where Rif intermediate was visible.
The authors extend their sincere thanks to Intermediate Reference Labs, State TB training and Demonstration Centre, Bangalore, Karnataka, India recognizing our research work and that successful completion of the work.
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Naik HJ, Bhuvaneshwari G. Phenotypic characterization and CBNAAT diagnosis of MTB from pulmonary and extra pulmonary samples. AGBIR.2023;39(6):695-697.
Received: 07-Oct-2023, Manuscript No. AGBIR-23-116077; , Pre QC No. AGBIR-23-116077 (PQ); Editor assigned: 10-Oct-2023, Pre QC No. AGBIR-23-116077 (PQ); Reviewed: 24-Oct-2023, QC No. AGBIR-23-116077; Revised: 31-Oct-2023, Manuscript No. AGBIR-23-116077 (R); Published: 07-Nov-2023, DOI: 10.35248/0970-1907.23.39.695-697
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
