Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Paper - (2023) Volume 39, Issue 5
Screening for resistance and their mechanism of resistance against gram pod borer Helicoverpa armigera (Hubner) conducted on chickpea during rabi season at Agriculture Research Farm, Institute of Agricultural Sciences, Banaras Hindu University Varanasi, India. Subsequently the laboratory experiment was conducted in the Department of Bio-chemistry, Institute of Science, Banaras Hindu University. Eighteen cultivars of chickpea genotypes were brought from Indian Institute of Pulses Research Kanpur and the genotypes were scaled for their relative resistance and susceptibility on a 1 to 9 scale while assessing these eighteen genotypes. This investigation of this study may be useful to know their mechanism of resistance against gram pod borer in chickpea.
Chickpea; Helicoverpa armigera; Resistance; Susceptibility; Screening
Pulses are the important component of food chain and feed system across the globe as they contribute immensely to food and nutritional security in sustainable manner through the diversification of agricultural production system. Chickpea (Cicer arietinum L.) is cultivated in almost all parts of world covering more than 50 countries spread over Asia, Africa, Europe, Australia, North America and South America. In India chickpea is grown almost in all parts of country mainly as rain fed crop. Madhya Pradesh is the single largest producer in the country. Although pulses comprise a number of different crops yet chickpea (Cicer arietinum L.) commonly known as gram is by far the most predominant member of this group. Chickpeas are one of the earliest known cultivated legumes, tracing their ancestry back at least 7,000 years to the dawn of agriculture. The crop is thought to have originated in southeast Turkey. Chickpea grain is an excellent source of high-quality protein, with a wide range of essential amino acids. The crop also fixes relatively large amounts of atmospheric nitrogen. Desi chickpeas are thought to be the earliest form of the crop. Kabuli chickpeas are grown largely in Southern Europe, Northern Africa, Afghanistan, Pakistan and Chile, but are also found on the Indian Subcontinent having been introduced there in the 18th century (ICRISAT). Though chickpea is a good source of protein, carbohydrate, dietary fiber, minerals (Fe, Zn, Ca, and Mg) and other important nutrients that are essential for human health, there is a scope for further improving nutritional quality of chickpea. Protein content of present day cultivars is usually in the range of 18-22%. In view of the known variation on susceptibility and resistance against Helicoverpa armigera among chickpea genotypes [1], the development of new cultivars which may less susceptible may offer better crop protection at present scenario. The problem of this pest is magnified due to its direct attack on fruiting structures, voracious feeding habit, high mobility and fecundity, multi-voltine nature, overlapping generations, nocturnal behavior and host selection by learning and propensity for acquiring resistance against pesticides.
The typical symptom of damage is half of the body hangs outside while head and thorax part inside the pod. Stout built, light brown female moth lay small white egg singly and mostly on the underside of leaflet. The eggs hatch in 3-4 days to produce yellowish caterpillar which attain a maximum length of about 3.5cm in 2-3 weeks under optimum conditions. The fully grown larvae bury themselves in the soil or among plant debris to pupate. In the summer and autumn, the pupal period lasts for about 6-12 days with total life cycle of about 4 weeks. The quantity of acid exudate imparts the resistance and susceptibility to chickpea against gram pod borer. The acid exudate is secreted from the glandular hairs which exudes droplet containing high concentration of Malic acid. Keeping in view some genotypes of known response were screened at Banaras Hindu University Uttar Pradesh India [2-4].
The experiment was conducted during 2015-16 and 2016-17 at Agriculture Research Farm, Banaras Hindu University, Varanasi. Eighteen chickpea cultivars were taken under screening trial. All recommended agronomic practices were adopted for the rabi season [5]. The genotypes were sown under insecticide free environment. The trial was sown in the first week of December in both the consecutive years in a randomized block design with three replications. The details of eighteen genotypes of chickpea are given in Table 1.
| Treatment No | Name | Treatment No | Name |
|---|---|---|---|
| T1 | PBG-5 | T10 | C-235(R) |
| T2 | RSG-931 | T11 | PUSA-261 |
| T3 | L-550 | T12 | RSG-10 |
| T4 | Radhey (LC) | T13 | CSJD-884 |
| T5 | PUSA-209 | T14 | GNG-1491 |
| T6 | GNG-581 | T15 | Digvijay |
| T7 | HC-3 | T16 | GCP-101 |
| T8 | GSJ-515 | T17 | AKGS-1 |
| T9 | GNG-146 | T18 | Annegiri-1(C) |
Table 1: The details of eighteen genotypes of chickpea.
The size of plot was 3 m × 1.80 m and spacing between rows to rows and plant to plant was 30 cm × 15 cm. Natural conditions was provided for the screening. Data on mean percentage of pods damaged by gram pod borer was recorded by selecting 5 plants randomly from each plot under consideration [6]. The cultivars of eighteen chickpeas were graded for their resistance and susceptibility as suggested by Lateef and Reed [1]. The relative acidity in the leaves of chickpea genotypes was estimated and correlated with the mean percentage of pods damaged by gram pod borer. The acidity of leaf exudates of 90 days old crop was estimated by titration method. Five leaves of chickpea were detached from every replication of genotype at 7.30 am morning, when maximum acid exudates are present for harvest. The detached leaves were kept in a small conical flask and washed with 30 ml of distilled water [7]. The water containing the acid was then titrated for acidity against NaOH solution using phenolphthalein as indicator. After that dry weight of leaves was determined by then for 3 days at 60°C. The mean of the two titration values adjusted for leaf weight, were then used to calculate mill equivalent of acidity for each genotype. The mean percentage of pods damage by Helicoverpa armigera at maturity was recorded by selecting five plants randomly from each plot under consideration. Radhey was taken as local check whereas Annegiri 1 was chosen as susceptible check. Whereas phenol and sugar was estimated by Singleton and Rossi and Dubois et al., protocols respectively [8].
Using a UV-VIS spectrophotometer for biochemical analysis of chickpea to screen for compounds like oxalic acid, malic acid, and phenols could provide valuable insights into the plant's potential defense mechanisms against insect pests like Helicoverpa armigera. Extracted oxalic acid from chickpea samples. This can be done by homogenizing chickpea tissues and extracting with a suitable solvent, followed by filtration.
Used the UV-VIS spectrophotometer to measure the absorbance of the extracted solution at a specific wavelength (e.g., 210 nm for oxalic acid) (Figure 1). Oxalic acid has a characteristic absorption peak at this wavelength. Prepared a standard curve using known concentrations of oxalic acid. The absorbance readings of the standards can be used to calculate the oxalic acid content in chickpea extracts. Extracted malic acid from chickpea samples using an appropriate method, such as acid hydrolysis, and dilute the extracts as needed. Measured the absorbance of the diluted extracts at a specific wavelength (e.g., 280 nm for malic acid). Malic acid exhibits absorbance at this wavelength. Created a standard curve using known concentrations of malic acid to quantify the malic acid content in chickpea samples (Figure 2).
Figure 1: Centrifuge machine and UV-VIS spectrophotometer for biochemical analysis.
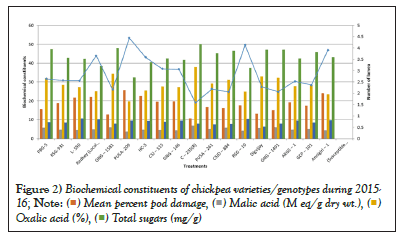
Figure 2: Biochemical constituents of chickpea varieties/genotypes during 2015-
16; 
Determined the total phenolic content by measuring the absorbance of the extract at a characteristic wavelength (e.g., 765 nm using the Folin-Ciocalteu reagent method). Phenols react with the Folin-Ciocalteu reagent, producing a color change that can be quantified spectrophotometrically. Performed statistical analysis to determine if there are significant differences in oxalic acid, malic acid, and phenol content between infested and non-infested chickpea samples. Correlated the biochemical data with insect damage or larval survival to assess the potential role of these compounds in chickpea defense against Helicoverpa armigera (Figure 3).
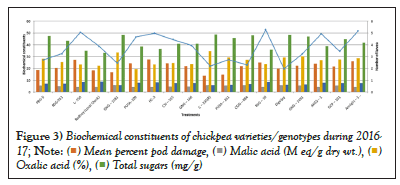
Figure 3: Biochemical constituents of chickpea varieties/genotypes during 2016-
17; 
Data and corresponding graph of year 2015-16 revealed that highest pod damage per cent was 25.62% in genotype Pusa-209 and the lowest pod damage per cent was 10.73% in genotype C-235 whereas the maximum mean number larval per meter row length was 4.44 in Pusa-209 and lowest was 1.58 in genotype C-235.For the next consecutive year 2016-17 the highest pod damage per cent was 27.52% in genotype HC-3 and the lowest pod damage per cent was 13.65% in genotype C-235 whereas the maximum mean number of larval per meter row length was 5.27 in RSG-10 and lowest was 1.97 in genotype Digvajay [9,10].
Data and corresponding graph presented in Table 2 shows that highest acidity of oxalic and malic acid was 6.86 per cent and 37.88 M eq/g dry wt. respectively in genotype C-235 in the year 2015-16. Whereas in year 2016-17 the highest acid exudate of oxalic and malic acid was 5.90 per cent and 34.52 M eq/g dry wt. respectively in genotype C-235 (Table 3) [11].
| Varieties/Genotypes | Mean no. of larvae per m-1 row | Mean per cent pod damage | Oxalic acid (%) | Malic acid (M eq/g dry wt.) |
|---|---|---|---|---|
| PBG-5 | 2.63 | 15.5 | 5.74 | 31.89 |
| RSG-931 | 2.57 | 18.65 | 4.79 | 28.46 |
| L-550 | 2.56 | 21.7 | 4.62 | 27.21 |
| Radhey (Local Check) | 3.66 | 22.19 | 4.9 | 24.97 |
| GNG-1581 | 2.16 | 12.86 | 5.88 | 34.23 |
| PUSA-209 | 4.44 | 25.62 | 3.83 | 19.72 |
| HC-3 | 3.59 | 22.54 | 4.78 | 25.41 |
| CSJ-515 | 3.08 | 19.62 | 4.57 | 27.67 |
| GNG-146 | 3.06 | 19.7 | 4.43 | 27.13 |
| C-235(R) | 1.58 | 10.73 | 6.86 | 37.88 |
| PUSA-261 | 2.19 | 16.77 | 5.13 | 29.26 |
| CSJD-884 | 2.06 | 16.26 | 5.64 | 31.24 |
| RSG-10 | 4.13 | 17.64 | 4.17 | 24.92 |
| Digvijay | 2.28 | 13.26 | 5.45 | 32.84 |
| GNG-1491 | 2.07 | 15.01 | 5.96 | 32.19 |
| AKGS-1 | 2.53 | 19.22 | 4.76 | 27.76 |
| GCP-101 | 2.36 | 17.27 | 5.1 | 28.31 |
| Annigiri-1 (Susceptible check) | 3.9 | 24.15 | 4.26 | 23.43 |
| SE(m)± | - | - | 0.27 | 0.31 |
| C.D.(0.05) | - | - | 0.84 | 1.01 |
Table 2: Biochemical constituents of chickpea varieties/genotypes during 2015-2016.
| Varieties/Genotypes | Mean no. of larvae per m-1 row | Mean per cent pod damage | Oxalic acid (%) | Malic acid (M eq/g dry wt.) |
|---|---|---|---|---|
| PBG-5 | 2.72 | 18.66 | 5.62 | 28.23 |
| RSG-931 | 3.25 | 20.38 | 4.94 | 25.32 |
| L-550 | 5.06 | 27.2 | 4.27 | 23.41 |
| Radhey (Local Check) | 3.87 | 18.29 | 4.2 | 22.27 |
| GNG-1581 | 2.44 | 16.87 | 5.7 | 33.6 |
| PUSA-209 | 4.66 | 24.2 | 4.38 | 19.29 |
| HC-3 | 4.98 | 27.52 | 4.32 | 23.19 |
| CSJ-515 | 4.45 | 24.16 | 4.52 | 24.6 |
| GNG-146 | 3.94 | 22.17 | 4.71 | 23.59 |
| C-235(R) | 2.2 | 13.65 | 5.9 | 34.52 |
| PUSA-261 | 2.73 | 14.85 | 5.5 | 29.33 |
| CSJD-884 | 2.29 | 21.87 | 5.78 | 27.3 |
| RSG-10 | 5.27 | 25 | 4.31 | 23.59 |
| Digvijay | 1.97 | 19.66 | 5.72 | 29.13 |
| GNG-1491 | 3.24 | 22.2 | 5.43 | 30.29 |
| AKGS-1 | 4.92 | 24.1 | 4.37 | 27.08 |
| GCP-101 | 3.46 | 21.75 | 5.41 | 27.59 |
| Annigiri-1 (Susceptible check) | 5.17 | 26.08 | 4.12 | 28.6 |
| SE(m)± | - | - | 0.29 | 0.05 |
| C.D.(0.05) | - | - | 0.78 | 0.18 |
Table 3: Biochemical constituents of chickpea varieties/genotypes during 2016-2017.
The correlation coefficient (r) of per cent pod damage with biochemical parameters during 2015-16 and 2016-17 shows negative correlation for the oxalic acid (-0.939** and -0.908**), malic acid (-0.958** and -0.877**) and total phenol (-0.944** and -0.951**) with per cent pod damage in both the experimental year. Whereas total sugar shows positive correlation with per cent pod damage (Table 4) [12].
| Oxalic acid | Malic acid | Phenol | Sugars | |
|---|---|---|---|---|
| % Pod damage 2016 | -0.939** | -0.958** | -0.944** | 0.915** |
| % Pod damage 2017 | -0.908** | -0.877** | -0.951** | 0.978** |
Note: * means significance at 5%.
Table 4: Correlation coefficient (r) of percent pod damage with biochemical parameters during 2015-16 and 2016-17.
Grain legumes play an important nutritional role in the diet of millions of people in the developing countries and are thus sometimes referred to as the poor man’s meat. Since legumes are vital sources of protein, calcium, iron, phosphorus, and other minerals, they form a significant part of the diet of vegetarians since the other food items they consume do not contain much protein. Above results show that genotype C-235 was less susceptible genotype and thus plant breeders in their breeding program for insect resistance can take this genotype as source of resistance against gram pod borer Helicoverpa armigera in chickpea. Further it is also clear from the above results that the genotype having low acidity in their leaf extract suffered more pod damage and vice versa. These results are in agreement with those of Rambold. The amount of acidity in leaf extract can therefore be used as index of the resistance/susceptibility of a genotype in chickpea.
[Crossref] [Google Scholar] [PubMed]
Citation: Narayan SR, Singh PS, Meena RS, et al. Screening for resistance and their mechanism of resistance against gram pod borer Helicoverpa armigera (Hubner) on Chickpea. AGBIR.2023;39(5):675-677.
Received: 17-Aug-2023, Manuscript No. AGBIR-23-113207 ; , Pre QC No. AGBIR-23-113207 (PQ); Editor assigned: 21-Aug-2023, Pre QC No. AGBIR-23-113207 (PQ); Reviewed: 04-Sep-2023, QC No. AGBIR-23-113207 ; Revised: 12-Sep-2023, Manuscript No. AGBIR-23-113207 (R); Published: 19-Sep-2023, DOI: 10.35248/0970-1907.23.39.675- 677
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
