Agricultural and Biological Research
RNI # 24/103/2012-R1
Review - (2021) Volume 37, Issue 1
Sugar beet (Beta vulgaris L.) is supplying approximately 35% of sugar worldwide. Rhizoctonia root rots in sugar beet is a setback for commercial cultivation. Disease severity increases when the weather is warm and under wet field conditions. Generally, integrated disease management strategies that involves cultural practice, chemical control, and host resistance are routinely followed to reduce the pathogen propagules. Alternatively, biocontrol strategies are environmentally safe, generally pose little risk of developing resistant biotypes, nevertheless, there has not been much success achieved in controlling R. solani in sugar beet with biocontrol agents in the field. This review article discussed the traditional management strategies and potential biological agents of Rhizoctonia solani.
tipobet vdcasino venüsbet sahabet sekabet sahabet onwin matadorbet casibom casibom casibom casinoplus casibom casibom jojobet jojobet jojobet grandpashabet grandpashabet grandpashabet
Sugar beet; Rhizoctonia solani; Rhizoctonia root
Sugar beet is an economically important crop of the large order Caryophyllales, supplying approximately 25% of sugar worldwide [1]. The sugar beet genome is diploid with 2n=18 chromosomes and the estimated genome size is 731 Mbp (megabases/millions of base pairs) [2]. The sugar beet wild ancestors are the sea beet (Beta vulgaris subsp. maritima) which resides in the family, Amaranthaceae and sub-family, Betoideae [3,4] (Figures 1 and 2). About 1500 years ago, sugar beet was introduced to China from Arabia. As it had high economic value in many countries, improvement of the crop has been extensively explored. It is a biennial crop with a sugar-rich tap root in the first year and a flowering seed stalk in the second. Currently, the crop is cultivated mainly in temperate regions between 30° and 60° N from Cairo to Helsinki [5,6].
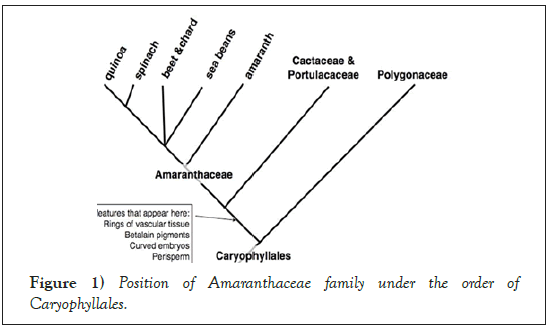
Figure 1: Position of Amaranthaceae family under the order of Caryophyllales.
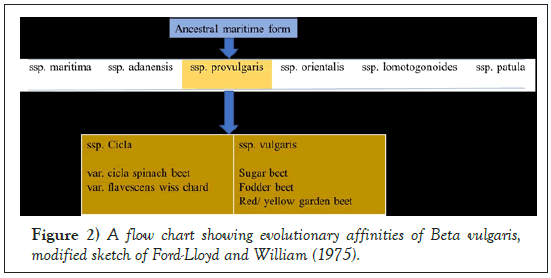
Figure 2: A flow chart showing evolutionary affinities of Beta vulgaris, modified sketch of Ford-Lloyd and William (1975).
In the USA, sugar beet production was done in 1838 in Northampton, MA, and the first successful sugar factory was set up in 1879, in Alvarado, CA, [7]. Sugar beet provides about 55% of the total sugar produced domestically, while sugar cane contributes 45% [8,9]. Sugar beet is currently grown in 11 states of the USA which includes Minnesota, Idaho, North Dakota, Michigan, Nebraska, Montana, California, Wyoming, Colorado, Oregon, and Washington. Sugar beet thrives well in temperate climatic conditions but can also be produced in warm climates.
The Red River Valley (RRV) of western Minnesota and eastern North Dakota is the largest area of producers of sugar beet in the United States. The first sugar beet factory was established in the RRV in 1926 in East Grand Forks [10]. Currently, there are three sugar beet cooperatives in the RRV: American Crystal Sugar Company, Minn-Dak Farmers’ Cooperative, and Southern Minnesota Beet Sugar Cooperative are located in Minnesota and North Dakota. These sugar beet cooperatives contribute approximately 57% of the US sugar beet acreage. This has created a huge economic impact of over $5 billion in the Upper Midwest. In USA, the total sugar beet planted area and yield was 1,132,000 acres and 28,600,000 tons, respectively in 2019.
Since the mid-1970s growers in the US started joining together as farm- owned cooperatives, purchased the processing companies and managed the marketing and sales of their production. In the USA, private companies own the commercial seed production and the variety improvement programs. The USDA helps to select and improve germplasm before making lines available to the seed companies for further development and commercialization. The varieties today are relatively high yielding and are moderately resistant to most of the common soil borne and foliar pathogens. For example, most varieties must have a minimum level of resistance to root pathogens such as Rhizoctonia, Aphanomyces, Pythium, Fusarium, sugar beet cyst nematode and viruses such as Beet Necrotic Yellow Vein and Curly Top [8,11,12]. Thus, the commercialization of sugar beet advanced with the establishment of sugar processing houses and increased in efficiency in the field and factory. Improved seed and varietal choice have made commercialization easier as it has helped farmers choose the best varieties for better yield and quality [13,14].
However, several diseases are major limiting factors to sugar beet yield potential. Cercospora leaf spot is one of the most important and widespread foliar diseases in sugar beet. It is caused by a hemibiotrophic filamentous fungal pathogen, Cercospora beticola, which causes necrotic lesions and progressive destruction of the plant’s foliage [6]. Research has been ongoing to map the genes that confer resistance to C. beticola [15]. Several other foliar diseases included alternaria leaf spot caused by Alternaria tenuissima, bacterial leaf spot caused by Pseudomonas syringae pv. aptata and powdery mildew caused by Erysiphe polygoni are occasionally observed in fields in the RRV [16-18].
Damping-off and root rots caused by A. cochlioides and R. solani are common diseases found in the RRV. Since 2009, R. solani, which causes damping-off and root rot, has been listed by growers in the RRV as one of their most important issues.
The Rhizoctonia genus was first reported by DeCandolle in 1815. This is known as a large, diverse and complex group of fungi. R. solani Kühn was first reported in 1858 by Julius Kühn who observed the fungal pathogen on potato tuber [19]. The basidiomycetes fungus belongs to the class Agaricomycetes, order Ceratobasidiales and the family Ceratobasidiaceae with a teleomorphic (Thanatephorus cucumeris [Frank] Donk) stage. R. solani is found in nature mainly in the asexual stage, and primarily prevalent form is vegetative mycelia and/or sclerotia [20-22]. The hyphae usually branch at a right angle with the presence of constriction at the base of the branch [23-25]. Fungal colony appears brown on potato dextrose agar (PDA) and amended clarified V8 media (CV8) (Figures 3 and 4).
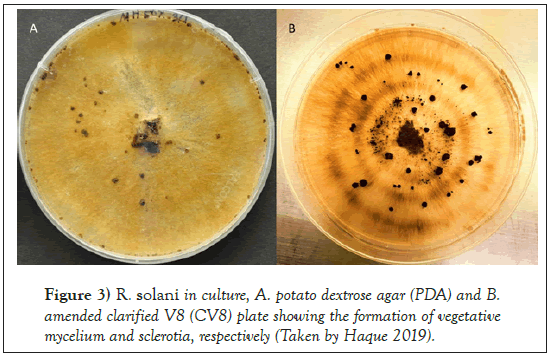
Figure 3: R. solani in culture, A. potato dextrose agar (PDA) and B. amended clarified V8 (CV8) plate showing the formation of vegetative mycelium and sclerotia, respectively (Taken by Haque 2019).
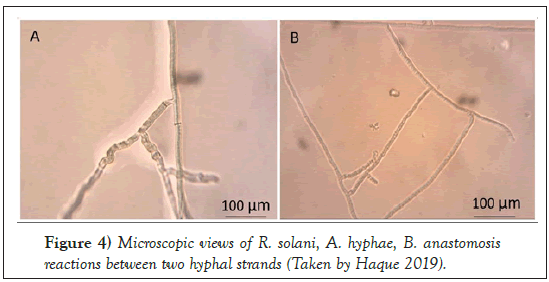
Figure 4: Microscopic views of R. solani, A. hyphae, B. anastomosis reactions between two hyphal strands (Taken by Haque 2019).
R. solani contains more than three nuclei per hyphal cell which contributes to significant heterozygosity within a single cell [26]. The heterokaryotic genome of R. solani covers approximately 51.7 Mbp and it is predicated to encode 12, 726 genes [27]. Rhizoctonia hyphae of several other species contains two nuclei known and are binucleate [28].
R. solani is a cosmopolitan, devastating soil-borne pathogen causing consistent economic losses in more than 200 plant species included a wide range of cereals, tubers, oilseed crops, and vegetables as well as ornamental plants and forest trees [29,25]. This facultative saprophyte has a variety of disease name based on crop plants; for instance, rice sheath blight, bare patch on cereals, black scurf on potatoes, sugar beet seedling damping-off, and crown and root rot, as well as damping-off, root and stem rot on soybean [11,30,31].
This pathogen undergoes hyphal fusion which is known as anastomosis. There are 13 anastomosis groups (AGs) [25,32]. Several studies in sugar beet plants have shown the presence of the following AGs: AG-1-1B, AG-1-1C, AG-2-1, AG-2-2, AG-4, AG-5, AG-11, AG-K, and AG-3TB [33-35]. Among these AGs, the most destructive form is AG-2-2 to sugar beet, which has two subgroups, AG-2-2 IIIB and AG-2-2 IV [36-38].
AG-2-2 IIIB was reported predominantly in the Red River Valley and in southern Minnesota, while AG 2-2 IV was rarely reported in past years [36]. AG 4 is mostly reported to cause post-damping-off of sugar beet [39]. AG-2- 2IIIB is aggressive to both seedlings and older sugar beet plants [3] (Figures 5 and 6). The annual yield loss varies from field to field, and state to state, ranging from 2% to 60% [40].
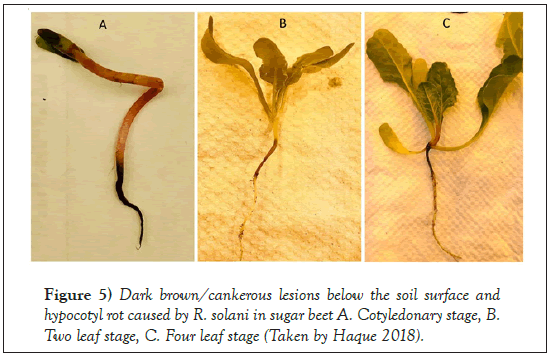
Figure 5: Dark brown/cankerous lesions below the soil surface and hypocotyl rot caused by R. solani in sugar beet A. Cotyledonary stage, B. Two leaf stage, C. Four leaf stage (Taken by Haque 2018).
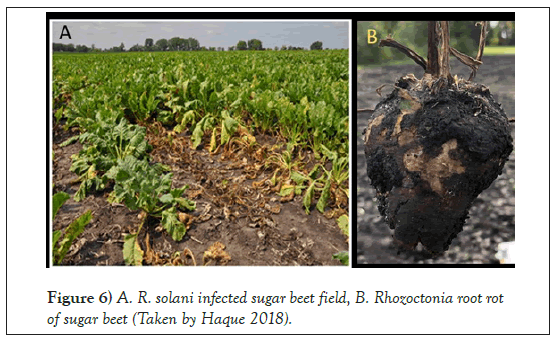
Figure 6: A. R. solani infected sugar beet field, B. Rhozoctonia root rot of sugar beet (Taken by Haque 2018).
R. solani can survive as sclerotia for a prolonged period in the soil. The conductive condition for infection depends on soil moisture (25%-100%) and soil temperature (20°C-35°C) [41]. Infections do not progress below 15°C [42]. The fungus can penetrate into the host through forming an infection cushion, or it can produce an appressorium that penetrates through the cell wall and take nutrients from the plant cell. The pathogen colonizes inside the dead tissue, and overwinter inside the host tissue as sclerotia (Figure 7) [43,33].
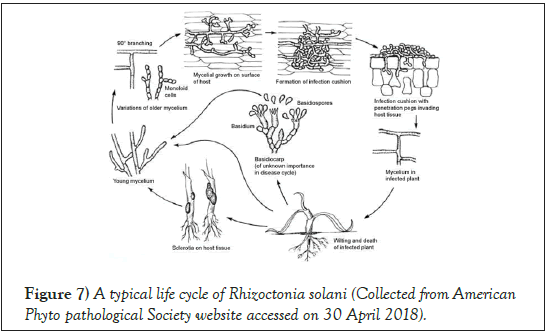
Figure 7: A typical life cycle of Rhizoctonia solani (Collected from American Phyto pathological Society website accessed on 30 April 2018).
This necrotrophic pathogen is also familiar as a seed and soil-borne fungus. The sign of infection appears as dark brown/cankerous lesions below the soil surface, and it advances to the hypocotyls. Symptoms may appear as yellowing or wilting of leaves. Sign and symptoms appeared on above and below ground portions of the plant. Consequently, it is yielding to the wilting and complete collapse of cotyledons and immature death of seedlings [11]. R. solani causes damping-off, and root and crown rot to young seedlings and older plants, respectively [8,42]. Root and crown rot in sugar beet incurs poor yield and infected plants become more susceptible to heat or drought stress [11].
R. solani damping-off as well as root and crown rot disease epidemics depend on the aggressiveness of the AGs, crops and cultivars, and the environment. Disease severity increases when the weather is warm and under wet field conditions. Generally, integrated pest management strategies that involves cultural practice, chemical control, host resistance and biological control are followed to reduce the pathogen propagules.
Cultural control
Cover crops studies have shown a significant effect in controlling Rhizoctonia infected fields. Cover crop (Brassica) used as green manure significantly reduced soil borne pathogens, particularly; Rhizoctonia, Phytohpthora, Pythium, Sclerotinia and Fusarium [44]. There is evidence that neem (Azadirachta indica) used as a green manure and Gliricidia leaves reduced inoculum of R. solani in paddy field by improving the microbial community structure [45]. However, cover crops are not commonly used in commercial fields to manage R. solani.
Agronomic tools such as crop rotation may help in reducing the disease. Sugar beet fields should be rotated at least every third year with non-host cereal crops such as wheat [46,47]; nevertheless, some AGs have the polyphagous nature to surmount this practice, for instance; AG 2-2IIIB strain of R. solani has a wide host range, including corn (Zea mays L.) and soybean (Glycine max L.) Merr.) [21]. Studies in Europe showed that AG 2-2 IIIB caused root and stalk root of corn [48]. Another study in south-eastern US demonstrated root and brace rot of corn was caused by R. solani AG 2-2 IIIB [49,50].
Similar study in the Upper Midwest showed that AG 2-2 IIIB caused disease lesions on corn in crop rotation studies which included wheat, soybean and corn [51]. This research suggested that cultivation of corn in crop rotation to sugar beet may escalate propagules of AG 2-2 IIIB. Likewise, AG8 has been reported aggressive to both cereal and legume rotations [52]. Also, AG1 and AG2 are aggressive to corn, canola, and soybean rotations [53,54]. Another study in New York has observed that crop rotation become very narrowly effective in controlling Rhizoctonia in table beets [55]. In practice, growers in the Red River Valley typically plant wheat, although it is not very profitable, as the crop preceding sugar beet to reduce the inoculum potential of R. solani. In several production areas of the US, such as southern Minnesota, producers grow the more profitable corn and soybean crops in rotation with sugar beet. Since both of these crops are host of R. solani AG2-2 IIIB, root rot has become more widespread and problematic where this rotation is common.
Improving soil structure and drainage is useful in improving water infiltration, drainage, and aeration of plants [41,46]. The availability of moderately resistant crop varieties with some reduction in yield is one way to avoid the significant yield loss in fields with a known history of severe disease [56]. Certified seed free from sclerotia can also reduce the chance of crop damage.
Chemical control
Fungicides such as azoxystrobin and pyraclostrobin (QoIs) and sedaxane, penthiopyrad, and fluxapyroxad (SDHIs) are widely used to control R. solani. The SDHI fungicides are typically used as fungicidal seed treatments while the QoIs may be applied at planting and foliarly (targeting the soil) to help control the pathogen [57-59].
Sugar beet growers prefer quinone outside inhibitors (QoI); azoxystrobin and pyraclostrobin. This helps to block electron transfer between cytochrome b and cytochrome c1 by binding to cytochrome b and it paves the halting of the ATP production [60]. These products are used as an in-furrow application at planting and as a foliar spray during the growing season [61,62]. QoI fungicides typically have a high risk for the buildup of a fungicide resistant pathogen subpopulation, particularly when used in fields with consecutive or repeated applications. Similarly, succinate dehydrogenase inhibitor (SDHI), for example, Penthiopyrad stops ATP production by binding to SDHI enzyme located in mitochondrial membrane [63]. Penthiopyrad and other SDHIs are used as a seed treatment [59]. Furthermore, demethylation inhibitor (DMI) such as Prothioconazole is a sterol biosynthesis that distrupts plasma membrane structure to incur abnormal fungal growth and death [64]. Greenhouse study at 26.7ºC showed that azoxystrobin and prothioconazole to be effective against R. solani AG2-2IIIB [65]. Producers in Minnesota and North Dakota indicated in an annual survey that the most commonly used fungicides were azoxystrobin, pyraclostrobin and prothioconazole to control R. solani [66].
The success of fungicide application depends on suitable timing that can offer long-term disease protection. Soil temperature and moisture are the two critical parameters that impact R. solani infection. Research shows that the mean daily soil temperature at the 10 cm soil depth needs to be at least 18 C for R. solani infection in sugar beet [41].
Host Resistance
Genetic resistance is an effective way of managing R. solani mediated diseases, as it involves low cost, effective, sustainable and an eco-friendly approach. Nevertheless, it takes 8-10 years to develop a resistant cultivar [67,68]. Sugar beet resistance breeding to Rhizoctonia started in 1950 at Fort Collins, Colorado by the United Sates Department of Agriculture-Agriculture Research Service (USDA-ARS). Sugar beet cultivars have moderate resistance to Rhizoctonia that involves multiple genes regulating the phenomenon of quantitative resistance [69,13,70]. Partial resistant varieties are grown to minimize the level of disease incidence, but growers prefer susceptible cultivars because of high yield potential [56,36].
In the US, private companies are mainly involved for developing resistant varieties, and in many cases, they do not disclose the genetic background of their resistant cultivars. Furthermore, the resistance of cultivars to R. solani is evaluated by scoring disease reactions at the crowns and roots of older seedlings [71], thus resistance is not evaluated during seed germination. Moreover, earlier studies evaluated cultivars resistance to R. solani using colonized whole barley or wheat grains which, unlike sclerotia, are artificial inocula of the pathogen that require time, space and technical know-how to produce. Moreover, colonized grains are prone to contamination with other pathogens and may be consumed by birds and wildlife when applied in the field. One of the objectives of this study was (1) to develop a medium for production of R. solani sclerotia, and compare the pathogenic potential of sclerotia, mycelia, and colonized barley grains to selected commercial sugar beet cultivars under greenhouse condition.
In the ecosystem, various types of interactions are occurring among all kinds of organisms, for instance; single-celled to multi-cellular organisms, pathogens are causing diseases while parasites are living on or in other living organisms to get their food [72]. On the other hand, symbiosis illustrates that the two organisms living together regardless of the outcome. However, there are a number of two species interactions in the nature that has been divided into two broad types, for example; negative interactions and positive interactions. There are a number of negative interactions in the ecosystem for example; parasitism, competition, amensalism, predation, and neutralism [73].
In parasitism, one species gets the benefits at the expense of the other, for example, bacteria, fungi, nematodes, and viruses. In competition both species competes with each other directly or indirectly for light, water, and food, for example, weeds in a crop [74-76]. In amensalism, one species is inhibited while other is unaffected, for example, many algae in nature. Another example is that bacteria-killing phenomenon of Penicillium [77]. In predation, there are a number of predatory insects in nature, for example, praying mantis that kills other insects. Neutralism is a type of interaction where neither population affects the other, for example, cacti and tarantulas living in the desert.
Apart from these negative interactions, there are a number of positive interactions available in nature, for example, mutualism, commensalism, and protocooperation [78].
The mutualism illustrates the favorable interactions to both species and it is obligatory, for example, lichens; a fungal partner (mycobiont) and algae (Cyanobacteria/Photobiont). Another example of cellulolytic bacteria harbored in the rumen of the cattle [79]. The commensalism phenomenon illustrates the interaction of species-1 (for example; orchids) which is directly benefited by the others, while species-2 is unaffected. For example, in the rain forest, orchids grown on the trees without causing any problem [80,81]. Protocooperation is another form of mutualism where interaction is favorable to both species but it is not obligatory. In this type of interaction occurs in soil bacteria or fungi and in plants growing in the soil [82].
As a component of integrated disease management, biocontrol strategies are environmentally safe, there is no chance of developing resistant biotypes, and it is convenient to use in greenhouse and field research.
Soil bacteria such as Rhizobacteria has shown the suppression effect on inoculum density of R. solani [83]. Similarly, a commercial preparation of Bacillus subtilis, Kodiak has been used to reduce R. solani AG 2-2 IIIB infection in sugar beet. Bacillus strain MSU-127 and low rate of Azoxystrobin used in- furrow application improved sugar beet by about 16% and a foliar fungicide application at the 4-leaf stage also increased root yield by 17% [84,85].
Antagonistic mechanisms of B. subtilis have been elucidated at the molecular level. For instance, B. subtilis produce bacteriocin which is a low molecular weight peptide molecule that involves different mode of action. This included protoplasm vesicularization, pore formation, and cell disintegration. Subtilin is the most studied bacteriocin that found to inhibit bacterial growth [86].
A wide diversity of secondary metabolites mediating antibiosis have been identified over the last two decades. Genome of most of the B. subtilis groups have revealed that 4-5% of genome devoted to antimicrobial peptides (AMPs) included ribosomal peptides (RPs) (bacteriocins and enzymes), the polyketides (PKs), the non-ribosomal peptides (NRPs) and volatile metabolites. Other types of enzymes are known to show antagonistic activities, quorum sensing, cell lysis or induction [87].
Recently, field application of B. subtilis was found to be effective for the control late blight of potato caused by Phytophthora infestans [88]. Other research showed that Bacillus velezensis LHSB1 strain controlled peanut stem rot caused by Sclerotium rolfsii [89]. Other greenhouse study on Bacillus amyloliquefaciens SB14 strain showed to reduce damping-off disease by 58% caused by R. solani AG-4 and 52% caused by R. solani AG 2-2 on sugar beet [90].
Fungus-like Laetisaria arvalis Burds. (Division: Basidiomycota, Order: Corticiales, Family: Corticiaceae) was reported as a potential biocontrol agent for soil-borne pathogens included Pythium species and R. solani [91]. In another research, Trichoderma harzianum in a conidial suspension has been used to inhibit the growth of R. solani and reduced disease index by 65% [92]. Research on the yeasts Candida valida, Trichosporon asahii and Rhodotorula glutinis protected sugar beet root rot from R. solani AG 2-2, and promoted plant growth in vitro [93]. Over the last four decades, several studies on biological control have been initiated, nevertheless, there has not been much success achieved in the field compared to the greenhouse. It is likely that very complex heterogenous biotic and abiotic factors influence the potential role of biocontrol agents.
The Penicillium is a large, diverse and ubiquitous genus that contains approximately 354 species. These are blue or green mold fungi that mostly exists as asexual (anamorph) stage. Some members of the genus are known to produce penicillin that is used as antibiotic to stop the growth of specific bacteria [94].
The Penicillium pinophilum Hedgc, belongs to the genus penicillium. This species was first reported in 1910 [95]. The synonymous name proposed as Talaromyces pinophilus [96]. This species belongs to Fungi, division Ascomycota, class Eurotiomycetes, order Eurotiales and family Aspergillaceae. The genome of P. pinophilum covers approximately 36.51 Mbp and it is predicated to encode 13, 472 protein-coding genes. Among the genes, 64 secondary metabolism gene clusters were annotated. In addition, 39 cellulose degrading and 24 starch degrading enzymes were identified [97]. This endophytic fungus is known to produce bioactive secondary metabolites including oxyskyrin, skyrin, dicatenarin, and 1,6,8-trihydroxy-3-hydroxy methylanthraquinone. These metabolites are involved to induce reactive oxygen species (ROS)-mediated apoptosis via mitochondrial pathway in cells [98]. This study demonstrated that P. pinophilum (T. pinophilus) produce useful biomass-degrading enzymes and secondary metabolites.
Other researchers found that P. pinophilum inoculation in soil increased nutrient uptake (N, P, and K) in pomegranate (Punica granatum L.) that resulted improved plant growth, significantly higher leaf area index and photosynthetic rate [99]. Research in India has shown that P. pinophilum can be used in biofertilizer formulation to supplement potassic fertilizer to pomegranate plant [100].
Another study has demonstrated the antagonistic potential of P. pinophilum and P. bilaiae in a dual-culture of phytopathogenic fungi including Alterniaria alternata, Fusarium equiseti, Fusarium graminearum, and Fusarium verticilloides [101]. This study demonstrated that the co-cultivation of plant beneficial fungi and phytopathogenic fungi may provide an effective strategy to simulate the production of bioactive metabolites, and thus possible help to identify novel compounds for crop protection.
Several studies on biocontrol of soilborne pathogens have been initiated, nevertheless, there has not been much success achieved in controlling R. solani in sugar beet. Lately, the biocontrol potential of Penicillium pinophilum (Talaromyces pinophilus) was reported to control Pythium and Rhizoctonia- induced damping-off in cucumber [102,103]. There are only a few fungicide chemistries which provide effective control of R. solani. Some countries do not allow the use of fungicides for control of R. solani in sugar beet. It will be useful to develop other novel ways to manage this important pathogen of sugar beet.
Citation: Haque ME, Parvin MS. Sugar beet, it ‘disease rhizoctonia root rot, and potential biological agents. AGBIR. 2021;37(1):96-101.
Received: 21-Dec-2020 Accepted: 04-Jan-2021 Published: 11-Jan-2021, DOI: 10.35248/0970-1907.21.37.96-101
